Page 58 - Read Online
P. 58
Page 6 of 21 Guo et al. Energy Mater. 2025, 5, 500041 https://dx.doi.org/10.20517/energymater.2024.214
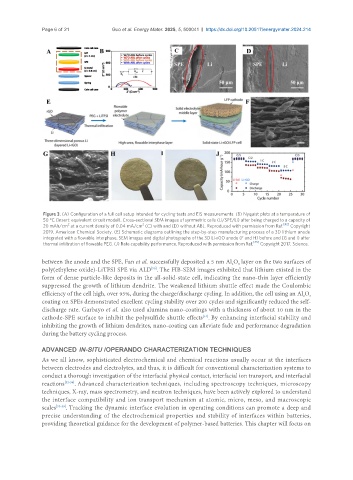
Figure 3. (A) Configuration of a full cell setup intended for cycling tests and EIS measurements. (B) Nyquist plots at a temperature of
50 °C (Insert: equivalent circuit model). Cross-sectional SEM images of symmetric cells (Li/SPE/Li) after being charged to a capacity of
2
2
20 mAh/cm at a current density of 0.04 mA/cm (C) with and (D) without ABL. Reproduced with permission from Ref. [42] Copyright
2019, American Chemical Society. (E) Schematic diagrams outlining the step-by-step manufacturing process of a 3D lithium anode
integrated with a flowable interphase. SEM images and digital photographs of the 3D Li-rGO anode (F and H) before and (G and I) after
thermal infiltration of flowable PEG. (J) Rate capability performance. Reproduced with permission from Ref. [45] Copyright 2017, Science.
between the anode and the SPE, Fan et al. successfully deposited a 5 nm Al O layer on the two surfaces of
3
2
poly(ethylene oxide)-LiTFSI SPE via ALD . The FIB-SEM images exhibited that lithium existed in the
[50]
form of dense particle-like deposits in the all-solid-state cell, indicating the nano-thin layer efficiently
suppressed the growth of lithium dendrite. The weakened lithium shuttle effect made the Coulombic
efficiency of the cell high, over 95%, during the charge/discharge cycling. In addition, the cell using an Al O
2
3
coating on SPEs demonstrated excellent cycling stability over 200 cycles and significantly reduced the self-
discharge rate. Garbayo et al. also used alumina nano-coatings with a thickness of about 10 nm in the
cathode-SPE surface to inhibit the polysulfide shuttle effects . By enhancing interfacial stability and
[51]
inhibiting the growth of lithium dendrites, nano-coating can alleviate fade and performance degradation
during the battery cycling process.
ADVANCED IN-SITU /OPERANDO CHARACTERIZATION TECHNIQUES
As we all know, sophisticated electrochemical and chemical reactions usually occur at the interfaces
between electrodes and electrolytes, and thus, it is difficult for conventional characterization systems to
conduct a thorough investigation of the interfacial physical contact, interfacial ion transport, and interfacial
reactions [52-54] . Advanced characterization techniques, including spectroscopy techniques, microscopy
techniques, X-ray, mass spectrometry, and neutron techniques, have been actively explored to understand
the interface compatibility and ion transport mechanism at atomic, micro, meso, and macroscopic
scales [55-59] . Tracking the dynamic interface evolution in operating conditions can promote a deep and
precise understanding of the electrochemical properties and stability of interfaces within batteries,
providing theoretical guidance for the development of polymer-based batteries. This chapter will focus on