Page 39 - Read Online
P. 39
Boaretto et al. Energy Mater. 2025, 5, 500040 https://dx.doi.org/10.20517/energymater.2024.203 Page 11 of 24
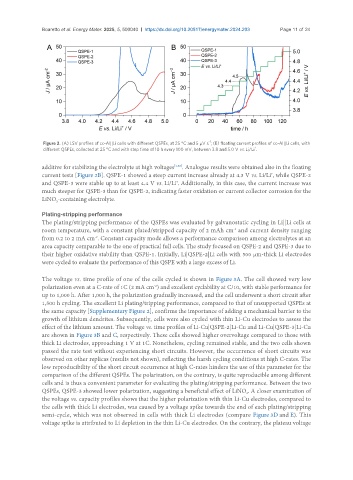
-1
Figure 2. (A) LSV profiles of cc-Al||Li cells with different QSPEs, at 25 °C and 5 µV s ; (B) floating current profiles of cc-Al||Li cells, with
+
different QSPEs, collected at 25 °C and with step time of 10 h every 100 mV, between 3.8 and 5.0 V vs. Li/Li .
additive for stabilizing the electrolyte at high voltages [1,38] . Analogue results were obtained also in the floating
current tests [Figure 2B]. QSPE-1 showed a steep current increase already at 4.3 V vs. Li/Li , while QSPE-2
+
+
and QSPE-3 were stable up to at least 4.4 V vs. Li/Li . Additionally, in this case, the current increase was
much steeper for QSPE-3 than for QSPE-2, indicating faster oxidation or current collector corrosion for the
LiNO -containing electrolyte.
3
Plating-stripping performance
The plating/stripping performance of the QSPEs was evaluated by galvanostatic cycling in Li||Li cells at
room temperature, with a constant plated/stripped capacity of 2 mAh cm and current density ranging
-2
-2
from 0.2 to 2 mA cm . Constant capacity mode allows a performance comparison among electrolytes at an
area capacity comparable to the one of practical full cells. The study focused on QSPE-2 and QSPE-3 due to
their higher oxidative stability than QSPE-1. Initially, Li|QSPE-2|Li cells with 500 µm-thick Li electrodes
were cycled to evaluate the performance of this QSPE with a large excess of Li.
The voltage vs. time profile of one of the cells cycled is shown in Figure 3A. The cell showed very low
-2
polarization even at a C-rate of 1C (2 mA cm ) and excellent cyclability at C/10, with stable performance for
up to 1,000 h. After 1,000 h, the polarization gradually increased, and the cell underwent a short circuit after
1,500 h cycling. The excellent Li plating/tripping performance, compared to that of unsupported QSPEs at
the same capacity [Supplementary Figure 2], confirms the importance of adding a mechanical barrier to the
growth of lithium dendrites. Subsequently, cells were also cycled with thin Li-Cu electrodes to assess the
effect of the lithium amount. The voltage vs. time profiles of Li-Cu|QSPE-2|Li-Cu and Li-Cu|QSPE-3|Li-Cu
are shown in Figure 3B and C, respectively. These cells showed higher overvoltage compared to those with
thick Li electrodes, approaching 1 V at 1C. Nonetheless, cycling remained stable, and the two cells shown
passed the rate test without experiencing short circuits. However, the occurrence of short circuits was
observed on other replicas (results not shown), reflecting the harsh cycling conditions at high C-rates. The
low reproducibility of the short circuit occurrence at high C-rates hinders the use of this parameter for the
comparison of the different QSPEs. The polarization, on the contrary, is quite reproducible among different
cells and is thus a convenient parameter for evaluating the plating/stripping performance. Between the two
QSPEs, QSPE-3 showed lower polarization, suggesting a beneficial effect of LiNO . A closer examination of
3
the voltage vs. capacity profiles shows that the higher polarization with thin Li-Cu electrodes, compared to
the cells with thick Li electrodes, was caused by a voltage spike towards the end of each plating/stripping
semi-cycle, which was not observed in cells with thick Li electrodes (compare Figure 3D and E). This
voltage spike is attributed to Li depletion in the thin Li-Cu electrodes. On the contrary, the plateau voltage