Page 38 - Read Online
P. 38
Page 10 of 24 Boaretto et al. Energy Mater. 2025, 5, 500040 https://dx.doi.org/10.20517/energymater.2024.203
Table 3. Selected transport parameters for supported QSPEs and Li|QSPE|Li cells: ionic conductivity at room temperature, Li +
transference number, Li/QSPE interface resistance in Li||Li cells, and steady-state current by applying a constant polarization ΔV of
10 mV
σ (25 °C)/mS cm -1 T + R /Ω cm 2 I /µA
*
ss
int
QSPE-1 1.2 ± 0.2 0.09 ± 0.01 240 ± 50 29 ± 5
QSPE-2 1.4 ± 0.3 0.12 ± 0.01 140 ± 20 47 ± 6
QSPE-3 1.2 ± 0.3 0.14 ± 0.01 107 ± 5 59 ± 2
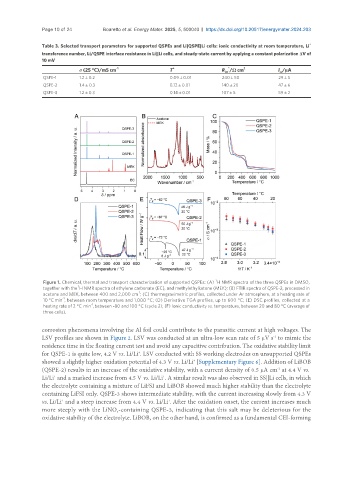
1
Figure 1. Chemical, thermal and transport characterization of supported QSPEs: (A) H NMR spectra of the three QSPEs in DMSO,
1
together with the H NMR spectra of ethylene carbonate (EC), and methylethylketone (MEK); (B) FTIR spectra of QSPE-2, processed in
-1
acetone and MEK, between 400 and 2,000 cm ; (C) thermogravimetric profiles, collected under Ar atmosphere, at a heating rate of
-1
10 °C min , between room temperature and 1,000 °C; (D) Derivative TGA profiles, up to 600 °C; (E) DSC profiles, collected at a
-1
heating rate of 2 °C min , between -80 and 100 °C (cycle 2); (F) Ionic conductivity vs. temperature, between 20 and 80 °C (average of
three cells).
corrosion phenomena involving the Al foil could contribute to the parasitic current at high voltages. The
-1
LSV profiles are shown in Figure 2. LSV was conducted at an ultra-low scan rate of 5 µV s to mimic the
residence time in the floating current test and avoid any capacitive contribution. The oxidative stability limit
+
for QSPE-1 is quite low, 4.2 V vs. Li/Li . LSV conducted with SS working electrodes on unsupported QSPEs
showed a slightly higher oxidation potential of 4.3 V vs. Li/Li [Supplementary Figure 6]. Addition of LiBOB
+
-2
(QSPE-2) results in an increase of the oxidative stability, with a current density of 0.5 µA cm at 4.4 V vs.
Li/Li and a marked increase from 4.5 V vs. Li/Li . A similar result was also observed in SS||Li cells, in which
+
+
the electrolyte containing a mixture of LiFSI and LiBOB showed much higher stability than the electrolyte
containing LiFSI only. QSPE-3 shows intermediate stability, with the current increasing slowly from 4.3 V
vs. Li/Li and a steep increase from 4.4 V vs. Li/Li . After the oxidation onset, the current increases much
+
+
more steeply with the LiNO -containing QSPE-3, indicating that this salt may be deleterious for the
3
oxidative stability of the electrolyte. LiBOB, on the other hand, is confirmed as a fundamental CEI-forming