Page 33 - Read Online
P. 33
Boaretto et al. Energy Mater. 2025, 5, 500040 https://dx.doi.org/10.20517/energymater.2024.203 Page 5 of 24
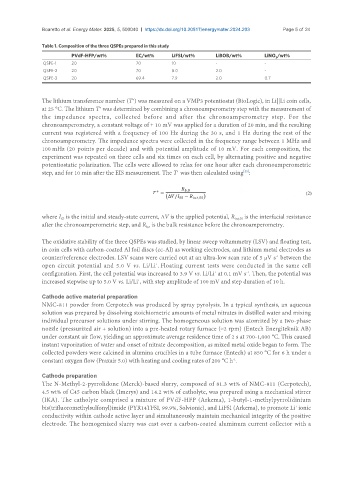
Table 1. Composition of the three QSPEs prepared in this study
PVdF-HFP/wt% EC/wt% LiFSI/wt% LiBOB/wt% LiNO /wt%
3
QSPE-1 20 70 10 - -
QSPE-2 20 70 8.0 2.0 -
QSPE-3 20 69.4 7.9 2.0 0.7
The lithium transference number (T ) was measured on a VMP3 potentiostat (BioLogic), in Li||Li coin cells,
+
+
at 25 °C. The lithium T was determined by combining a chronoamperometry step with the measurement of
the impedance spectra, collected before and after the chronoamperometry step. For the
chronoamperometry, a constant voltage of ± 10 mV was applied for a duration of 20 min, and the resulting
current was registered with a frequency of 100 Hz during the 30 s, and 1 Hz during the rest of the
chronoamperometry. The impedance spectra were collected in the frequency range between 1 MHz and
100 mHz (20 points per decade) and with potential amplitude of 10 mV. For each composition, the
experiment was repeated on three cells and six times on each cell, by alternating positive and negative
potentiostatic polarization. The cells were allowed to relax for one hour after each chronoamperometric
step, and for 10 min after the EIS measurement. The T was then calculated using :
[55]
+
where I is the initial and steady-state current, ΔV is the applied potential, R int,SS is the interfacial resistance
SS
after the chronoamperometric step, and R is the bulk resistance before the chronoamperometry.
b,0
The oxidative stability of the three QSPEs was studied, by linear sweep voltammetry (LSV) and floating test,
in coin cells with carbon-coated Al foil discs (cc-Al) as working electrodes, and lithium metal electrodes as
counter/reference electrodes. LSV scans were carried out at an ultra-low scan rate of 5 µV s between the
-1
open circuit potential and 5.0 V vs. Li/Li . Floating current tests were conducted in the same cell
+
configuration. First, the cell potential was increased to 3.9 V vs. Li/Li at 0.1 mV s . Then, the potential was
-1
+
increased stepwise up to 5.0 V vs. Li/Li , with step amplitude of 100 mV and step duration of 10 h.
+
Cathode active material preparation
NMC-811 powder from Cerpotech was produced by spray pyrolysis. In a typical synthesis, an aqueous
solution was prepared by dissolving stoichiometric amounts of metal nitrates in distilled water and mixing
individual precursor solutions under stirring. The homogeneous solution was atomized by a two-phase
nozzle (pressurized air + solution) into a pre-heated rotary furnace (≈2 rpm) (Entech Energiteknik AB)
under constant air flow, yielding an approximate average residence time of 2 s at 700-1,000 °C. This caused
instant vaporization of water and onset of nitrate decomposition, as mixed metal oxide began to form. The
collected powders were calcined in alumina crucibles in a tube furnace (Entech) at 850 °C for 6 h under a
constant oxygen flow (Praxair 5.0) with heating and cooling rates of 200 °C h .
-1
Cathode preparation
The N-Methyl-2-pyrrolidone (Merck)-based slurry, composed of 81.3 wt% of NMC-811 (Cerpotech),
4.5 wt% of C45 carbon black (Imerys) and 14.2 wt% of catholyte, was prepared using a mechanical stirrer
(IKA). The catholyte comprised a mixture of PVdF-HFP (Arkema), 1-butyl-1-methylpyrrolidinium
+
bis(trifluoromethylsulfonyl)imide (PYR14TFSI, 99.9%, Solvionic), and LiFSI (Arkema), to promote Li ionic
conductivity within cathode active layer and simultaneously maintain mechanical integrity of the positive
electrode. The homogenized slurry was cast over a carbon-coated aluminum current collector with a