Page 191 - Read Online
P. 191
Cui et al. Energy Mater 2023;3:300023 https://dx.doi.org/10.20517/energymater.2022.90 Page 7 of 12
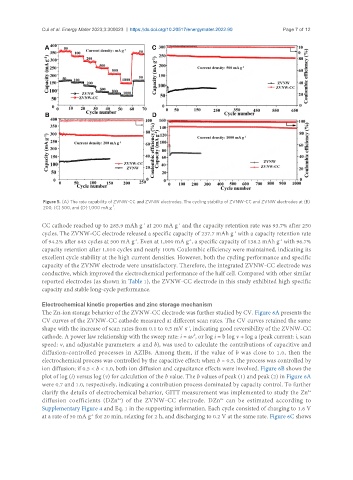
Figure 5. (A) The rate capability of ZVNW-CC and ZVNW electrodes. The cycling stability of ZVNW-CC and ZVNW electrodes at (B)
-1
200, (C) 500, and (D) 1,000 mA g .
-1
CC cathode reached up to 285.9 mAh g at 200 mA g and the capacity retention rate was 93.7% after 250
-1
cycles. The ZVNW-CC electrode released a specific capacity of 237.7 mAh g with a capacity retention rate
-1
-1
-1
-1
of 94.2% after 645 cycles at 500 mA g . Even at 1,000 mA g , a specific capacity of 138.2 mAh g with 96.7%
capacity retention after 1,010 cycles and nearly 100% Coulombic efficiency were maintained, indicating its
excellent cycle stability at the high current densities. However, both the cycling performance and specific
capacity of the ZVNW electrode were unsatisfactory. Therefore, the integrated ZVNW-CC electrode was
conductive, which improved the electrochemical performance of the half cell. Compared with other similar
reported electrodes (as shown in Table 1), the ZVNW-CC electrode in this study exhibited high specific
capacity and stable long-cycle performance.
Electrochemical kinetic properties and zinc storage mechanism
The Zn-ion storage behavior of the ZVNW-CC electrode was further studied by CV. Figure 6A presents the
CV curves of the ZVNW-CC cathode measured at different scan rates. The CV curves retained the same
shape with the increase of scan rates from 0.1 to 0.5 mV s , indicating good reversibility of the ZVNW-CC
-1
cathode. A power law relationship with the sweep rate: i = av , or log i = b log v + log a (peak current: i, scan
b
speed: v, and adjustable parameters: a and b), was used to calculate the contributions of capacitive and
diffusion-controlled processes in AZIBs. Among them, if the value of b was close to 1.0, then the
electrochemical process was controlled by the capacitive effect; when b = 0.5, the process was controlled by
ion diffusion; if 0.5 < b < 1.0, both ion diffusion and capacitance effects were involved. Figure 6B shows the
plot of log (i) versus log (v) for calculation of the b value. The b values of peak (1) and peak (2) in Figure 6A
were 0.7 and 1.0, respectively, indicating a contribution process dominated by capacity control. To further
clarify the details of electrochemical behavior, GITT measurement was implemented to study the Zn
2+
diffusion coefficients (DZn ) of the ZVNW-CC electrode. DZn can be estimated according to
2+
2+
Supplementary Figure 4 and Eq. 1 in the supporting information. Each cycle consisted of charging to 1.6 V
at a rate of 50 mA g for 20 min, relaxing for 2 h, and discharging to 0.2 V at the same rate. Figure 6C shows
-1