Page 19 - Read Online
P. 19
Page 4 of 35 Tao et al. Energy Mater 2022;2:200036 https://dx.doi.org/10.20517/energymater.2022.46
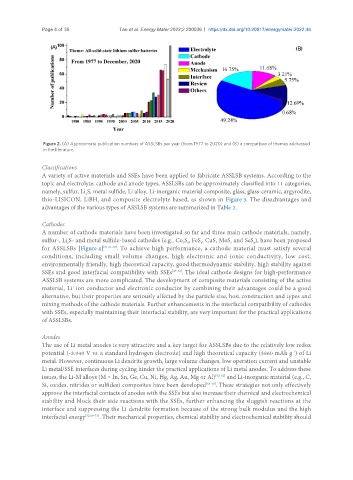
Figure 2. (A) Approximate publication numbers of ASSLSBs per year (from 1977 to 2020) and (B) a comparison of themes addressed
in the literature.
Classifications
A variety of active materials and SSEs have been applied to fabricate ASSLSB systems. According to the
topic and electrolyte, cathode and anode types, ASSLSBs can be approximately classified into 11 categories,
namely, sulfur, Li S, metal sulfide, Li alloy, Li-inorganic material composite, glass, glass-ceramic, argyrodite,
2
thio-LISICON, LiBH and composite electrolyte based, as shown in Figure 3. The disadvantages and
4
advantages of the various types of ASSLSB systems are summarized in Table 2.
Cathodes
A number of cathode materials have been investigated so far and three main cathode materials, namely,
sulfur-, Li S- and metal sulfide-based cathodes (e.g., Co S , FeS , CuS, MoS and SeS ), have been proposed
2
9 8
3
x
x
for ASSLSBs [Figure 4] [31,41-46] . To achieve high performance, a cathode material must satisfy several
conditions, including small volume changes, high electronic and ionic conductivity, low cost,
environmentally friendly, high theoretical capacity, good thermodynamic stability, high stability against
SSEs and good interfacial compatibility with SSEs [47-52] . The ideal cathode designs for high-performance
ASSLSB systems are more complicated. The development of composite materials consisting of the active
+
material, Li ion conductor and electronic conductor by combining their advantages could be a good
alternative, but their properties are seriously affected by the particle size, host construction and types and
mixing methods of the cathode materials. Further enhancements in the interfacial compatibility of cathodes
with SSEs, especially maintaining their interfacial stability, are very important for the practical applications
of ASSLSBs.
Anodes
The use of Li metal anodes is very attractive and a key target for ASSLSBs due to the relatively low redox
potential (-3.040 V vs. a standard hydrogen electrode) and high theoretical capacity (3860 mAh g ) of Li
-1
metal. However, continuous Li dendrite growth, large volume changes, low operation current and unstable
Li metal/SSE interfaces during cycling hinder the practical applications of Li metal anodes. To address these
issues, the Li-M alloys (M = In, Sn, Ge, Cu, Ni, Hg, Ag, Au, Mg or Al) [53-62] and Li-inorganic material (e.g., C,
Si, oxides, nitrides or sulfides) composites have been developed [63-68] . These strategies not only effectively
approve the interfacial contacts of anodes with the SSEs but also increase their chemical and electrochemical
stability and block their side reactions with the SSEs, further enhancing the sluggish reactions at the
interface and suppressing the Li dendrite formation because of the strong bulk modulus and the high
interfacial energy [32,69-73] . Their mechanical properties, chemical stability and electrochemical stability should