Page 133 - Read Online
P. 133
Xiao et al. Energy Mater 2023;3:300007 https://dx.doi.org/10.20517/energymater.2022.84 Page 7 of 13
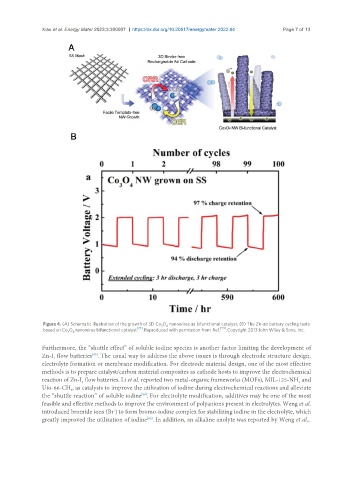
Figure 4. (A) Schematic illustration of the growth of 3D Co O nanowires as bifunctional catalyst; (B) The Zn-air battery cycling tests
3
4
based on Co O nanowires bifunctional catalyst [74] . Reproduced with permission from Ref. [74] . Copyright 2013 John Wiley & Sons, Inc.
3
4
Furthermore, the “shuttle effect” of soluble iodine species is another factor limiting the development of
Zn-I flow batteries . The usual way to address the above issues is through electrode structure design,
[85]
2
electrolyte formation or membrane modification. For electrode material design, one of the most effective
methods is to prepare catalyst/carbon material composites as cathode hosts to improve the electrochemical
reaction of Zn-I flow batteries. Li et al. reported two metal-organic frameworks (MOFs), MIL-125-NH and
2
2
Uio-66-CH , as catalysts to improve the utilization of iodine during electrochemical reactions and alleviate
3
the “shuttle reaction” of soluble iodine . For electrolyte modification, additives may be one of the most
[89]
feasible and effective methods to improve the environment of polyanions present in electrolytes. Weng et al.
introduced bromide ions (Br ) to form bromo-iodine complex for stabilizing iodine in the electrolyte, which
-
greatly improved the utilization of iodine . In addition, an alkaline anolyte was reported by Weng et al.,
[90]