Page 130 - Read Online
P. 130
Page 4 of 13 Xiao et al. Energy Mater 2023;3:300007 https://dx.doi.org/10.20517/energymater.2022.84
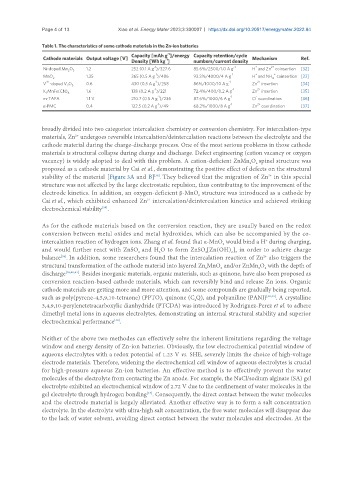
Table 1. The characteristics of some cathode materials in the Zn-ion batteries
-1
Capacity [mAh g ]/energy Capacity retention/cycle
Cathode materials Output voltage [V] -1 Mechanism Ref.
Density [Wh kg ] numbers/current density
-1 -1 + 2+
Ni-doped Mn O 1.2 252 (0.1 A g )/327.6 85.6%/2500/1.0 A g H and Zn coinsertion [32]
2 3
+
-1
+
MnO 2 1.35 365 (0.5 A g )/486 93.3%/4000/4 A g -1 H and NH coinsertion [33]
4
4+ -1 -1 2+
V -doped V O 0.6 430 (0.5 A g )/258 86%/1000/10 A g Zn insertion [34]
2 5
-1
2+
K MnFe(CN) 6 1.6 138 (0.2 A g )/221 72.4%/400/0.2 A g -1 Zn insertion [35]
2
-1 -1 -
m-TAPA 1.1 V 210.7 (0.5 A g )/236 87.6%/1000/6 A g Cl coordination [36]
-1 -1 2+
π-PMC 0.4 122.5 (0.2 A g )/49 68.2%/1000/8 A g Zn coordination [37]
broadly divided into two categories: intercalation chemistry or conversion chemistry. For intercalation-type
2+
materials, Zn undergoes reversible intercalation/deintercalation reactions between the electrolyte and the
cathode material during the charge-discharge process. One of the most serious problems in those cathode
materials is structural collapse during charge and discharge. Defect engineering (cation vacancy or oxygen
vacancy) is widely adopted to deal with this problem. A cation-deficient ZnMn O spinel structure was
4
2
proposed as a cathode material by Cai et al., demonstrating the positive effect of defects on the structural
2+
[38]
stability of the material [Figure 3A and B] . They believed that the migration of Zn in this special
structure was not affected by the large electrostatic repulsion, thus contributing to the improvement of the
electrode kinetics. In addition, an oxygen-deficient β-MnO structure was introduced as a cathode by
2
Cai et al., which exhibited enhanced Zn intercalation/deintercalation kinetics and achieved striking
2+
[39]
electrochemical stability .
As for the cathode materials based on the conversion reaction, they are usually based on the redox
conversion between metal oxides and metal hydroxides, which can also be accompanied by the co-
intercalation reaction of hydrogen ions. Zhang et al. found that α-MnO would bind a H during charging,
+
2
and would further react with ZnSO and H O to form ZnSO [Zn(OH) ] in order to achieve charge
4
4
2 3
2
balance . In addition, some researchers found that the intercalation reaction of Zn also triggers the
2+
[38]
structural transformation of the cathode material into layered Zn MnO and/or ZnMn O with the depth of
x
4
2
2
discharge [28,40,41] . Besides inorganic materials, organic materials, such as quinone, have also been proposed as
conversion reaction-based cathode materials, which can reversibly bind and release Zn ions. Organic
cathode materials are getting more and more attention, and some compounds are gradually being reported,
such as poly(pyrene-4,5,9,10-tetraone) (PPTO), quinone (C Q), and polyaniline (PANI) [30,42] . A crystalline
4
3,4,9,10-perylenetetracarboxylic dianhydride (PTCDA) was introduced by Rodriguez-Perez et al. to adhere
dimethyl metal ions in aqueous electrolytes, demonstrating an internal structural stability and superior
[43]
electrochemical performance .
Neither of the above two methodes can effectively solve the inherent limitations regarding the voltage
window and energy density of Zn-ion batteries. Obviously, the low electrochemical potential window of
aqueous electrolytes with a redox potential of 1.23 V vs. SHE, severely limits the choice of high-voltage
electrode materials. Therefore, widening the electrochemical cell window of aqueous electrolytes is crucial
for high-pressure aqueous Zn-ion batteries. An effective method is to effectively prevent the water
molecules of the electrolyte from contacting the Zn anode. For example, the NaCl/sodium alginate (SA) gel
electrolyte exhibited an electrochemical window of 2.72 V due to the confinement of water molecules in the
[44]
gel electrolyte through hydrogen bonding . Consequently, the direct contact between the water molecules
and the electrode material is largely alleviated. Another effective way is to form a salt concentration
electrolyte. In the electrolyte with ultra-high salt concentration, the free water molecules will disappear due
to the lack of water solvent, avoiding direct contact between the water molecules and electrodes. At the