Page 12 - Read Online
P. 12
Page 8 of 11 Chen et al. Energy Mater 2022;2:200033 https://dx.doi.org/10.20517/energymater.2022.36
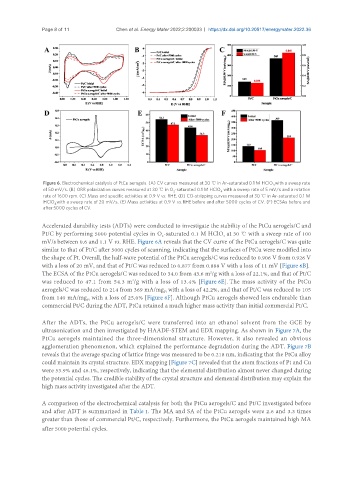
Figure 6. Electrochemical catalysis of PtCu aerogels. (A) CV curves measured at 30 ℃ in Ar-saturated 0.1 M HClO with a sweep rate
4
of 50 mV/s. (B) ORR polarization curves measured at 30 ℃ in O -saturated 0.1 M HClO with a sweep rate of 5 mV/s and a rotation
2 4
rate of 1600 rpm. (C) Mass and specific activities at 0.9 V vs. RHE. (D) CO-stripping curves measured at 30 ℃ in Ar-saturated 0.1 M
HClO with a sweep rate of 20 mV/s. (E) Mass activities at 0.9 V vs. RHE before and after 5000 cycles of CV. (F) ECSAs before and
4
after 5000 cycles of CV.
Accelerated durability tests (ADTs) were conducted to investigate the stability of the PtCu aerogels/C and
Pt/C by performing 5000 potential cycles in O -saturated 0.1 M HClO at 30 ℃ with a sweep rate of 100
4
2
mV/s between 0.6 and 1.1 V vs. RHE. Figure 6A reveals that the CV curve of the PtCu aerogels/C was quite
similar to that of Pt/C after 5000 cycles of scanning, indicating that the surfaces of PtCu were modified into
the shape of Pt. Overall, the half-wave potential of the PtCu aerogels/C was reduced to 0.906 V from 0.926 V
with a loss of 20 mV, and that of Pt/C was reduced to 0.877 from 0.888 V with a loss of 11 mV [Figure 6B].
The ECSA of the PtCu aerogels/C was reduced to 34.0 from 43.6 m /g with a loss of 22.1%, and that of Pt/C
2
was reduced to 47.1 from 54.3 m /g with a loss of 13.4% [Figure 6E]. The mass activity of the PtCu
2
aerogels/C was reduced to 214 from 369 mA/mg with a loss of 42.2%, and that of Pt/C was reduced to 105
Pt
from 140 mA/mg with a loss of 25.0% [Figure 6F]. Although PtCu aerogels showed less endurable than
Pt
commercial Pt/C during the ADT, PtCu retained a much higher mass activity than initial commercial Pt/C.
After the ADTs, the PtCu aerogels/C were transferred into an ethanol solvent from the GCE by
ultrasonication and then investigated by HAADF-STEM and EDX mapping. As shown in Figure 7A, the
PtCu aerogels maintained the three-dimensional structure. However, it also revealed an obvious
agglomeration phenomenon, which explained the performance degradation during the ADT. Figure 7B
reveals that the average spacing of lattice fringe was measured to be 0.218 nm, indicating that the PtCu alloy
could maintain its crystal structure. EDX mapping [Figure 7C] revealed that the atom fractions of Pt and Cu
were 53.9% and 46.1%, respectively, indicating that the elemental distribution almost never changed during
the potential cycles. The credible stability of the crystal structure and elemental distribution may explain the
high mass activity investigated after the ADT.
A comparison of the electrochemical catalysis for both the PtCu aerogels/C and Pt/C investigated before
and after ADT is summarized in Table 1. The MA and SA of the PtCu aerogels were 2.6 and 3.3 times
greater than those of commercial Pt/C, respectively. Furthermore, the PtCu aerogels maintained high MA
after 5000 potential cycles.