Page 11 - Read Online
P. 11
Chen et al. Energy Mater 2022;2:200033 https://dx.doi.org/10.20517/energymater.2022.36 Page 7 of 11
Figure 4. Structural characterization of PtCu(CS) aerogels. (A, B) SEM, (C) TEM and (D) HRTEM images. The inset in (C) is the SAED
pattern recorded from (C). The inset in (D) is the corresponding FFT pattern of panel (D).
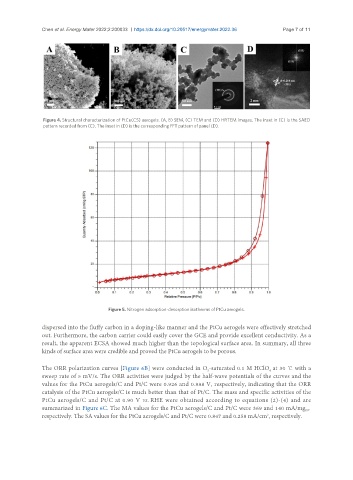
Figure 5. Nitrogen adsorption-desorption isotherms of PtCu aerogels.
dispersed into the fluffy carbon in a doping-like manner and the PtCu aerogels were effectively stretched
out. Furthermore, the carbon carrier could easily cover the GCE and provide excellent conductivity. As a
result, the apparent ECSA showed much higher than the topological surface area. In summary, all three
kinds of surface area were credible and proved the PtCu aerogels to be porous.
The ORR polarization curves [Figure 6B] were conducted in O -saturated 0.1 M HClO at 30 ℃ with a
2
4
sweep rate of 5 mV/s. The ORR activities were judged by the half-wave potentials of the curves and the
values for the PtCu aerogels/C and Pt/C were 0.926 and 0.888 V, respectively, indicating that the ORR
catalysis of the PtCu aerogels/C is much better than that of Pt/C. The mass and specific activities of the
PtCu aerogels/C and Pt/C at 0.90 V vs. RHE were obtained according to equations (2)-(4) and are
summarized in Figure 6C. The MA values for the PtCu aerogels/C and Pt/C were 369 and 140 mA/mg ,
Pt
respectively. The SA values for the PtCu aerogels/C and Pt/C were 0.847 and 0.258 mA/cm , respectively.
2