Page 22 - Read Online
P. 22
Dang et al. Chem Synth 2023;3:14 https://dx.doi.org/10.20517/cs.2022.33 Page 3 of 20
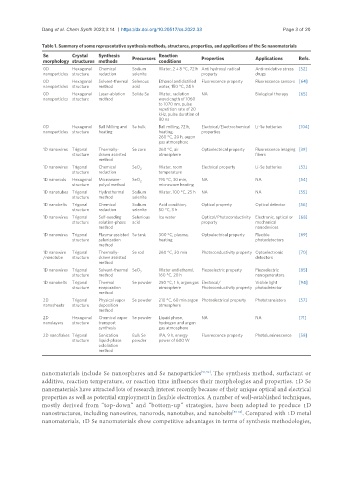
Table 1. Summary of some representative synthesis methods, structures, properties, and applications of the Se nanomaterials
Se Crystal Synthesis Precursors Reaction Properties Applications Refs.
morphology structures methods conditions
0D Hexagonal Chemical Sodium Water, 2 ± 8 °C, 72 h Anti hydroxyl radical Anti-oxidative stress [52]
nanoparticles structure reduction selenite property drugs
0D Hexagonal Solvent-thermal Selenous Ethanol and distilled Fluorescence property Fluorescence sensors [64]
nanoparticles structure method acid water, 150 °C, 24 h
0D Hexagonal Laser-ablation Solide Se Water, radiation NA Biological therapy [65]
nanoparticles structure method wavelength of 1060
to 1070 nm, pulse
repetition rate of 20
kHz, pulse duration of
80 ns
0D Hexagonal Ball Milling and Se bulk Ball milling, 72 h, Electrical/Electrochemical Li-Se batteries [104]
nanoparticles structure heating heating, properties
260 °C, 20 h, argon
gas atmosphere
1D nanowires Trigonal Thermally- Se core 260 °C, air Optoelectrical property Fluorescence imaging [39]
structure drawn assisted atmosphere fibers
method
1D nanowires Trigonal Chemical SeO Water, room Electrical property Li-Se batteries [53]
2
structure reduction temperature
1D nanorods Hexagonal Microwave- SeO 2 195 °C, 30 min, NA NA [54]
structure polyol method microwave heating
1D nanotubes Trigonal Hydrothermal Sodium Water, 100 °C, 25 h NA NA [55]
structure method selenite
1D nanobelts Trigonal Chemical Sodium Acid condition, Optical property Optical detector [56]
structure reduction selenite 50 °C, 3 h
1D nanowires Trigonal Self-seeding Selenious Ice water Optical/Photoconductivity Electronic, optical or [68]
structure solution-phase acid property mechanical
method nanodevices
1D nanowires Trigonal Plasma-assisted Se tank 300 °C, plasma, Optoelectrical property Flexible [69]
structure selenization heating photodetectors
method
1D nanowire Trigonal Thermally- Se rod 260 °C, 30 min Photoconductivity property Optoelectronic [70]
/nanotube structure drawn assisted detectors
method
1D nanowires Trigonal Solvent-thermal SeO 2 Water and ethanol, Piezoelectric property Piezoelectric [85]
structure method 160 °C, 20 h nanogenerators
1D nanobelts Trigonal Thermal Se powder 250 °C, 1 h, argon gas Electrical/ Visible light [94]
structure evaporation atmosphere Photoconductivity property photodetector
method
2D Trigonal Physical vapor Se powder 210 °C, 60 min argon Photoelectrical property Phototransistors [57]
nanosheets structure deposition atmosphere
method
2D Hexagonal Chemical vapor Se powder Liquid phase, NA NA [71]
nanolayers structure transport hydrogen and argon
synthesis gas atmosphere
2D nanoflakes Trigonal Sonication Bulk Se IPA, 9 h, energy Fluorescence property Photoluminescence [58]
structure liquid-phase powder power of 600 W
exfoliation
method
nanomaterials include Se nanospheres and Se nanoparticles [51,52] . The synthesis method, surfactant or
additive, reaction temperature, or reaction time influences their morphologies and properties. 1D Se
nanomaterials have attracted lots of research interest recently because of their unique optical and electrical
properties as well as potential employment in flexible electronics. A number of well-established techniques,
mostly derived from “top-down” and “bottom-up” strategies, have been adopted to produce 1D
nanostructures, including nanowires, nanorods, nanotubes, and nanobelts [53-56] . Compared with 1D metal
nanomaterials, 1D Se nanomaterials show competitive advantages in terms of synthesis methodologies,