Page 36 - Read Online
P. 36
Lee et al. Cancer Drug Resist 2020;3:980-91 I http://dx.doi.org/10.20517/cdr.2020.73 Page 987
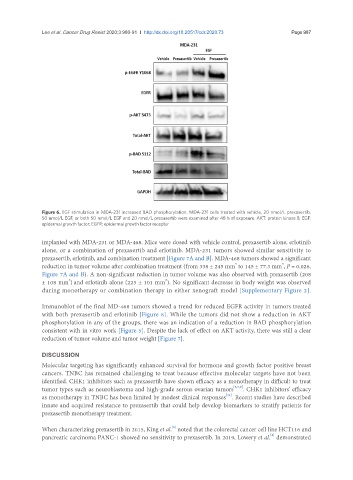
Figure 6. EGF stimulation in MDA-231 increased BAD phosphorylation. MDA-231 cells treated with vehicle, 20 nmol/L prexasertib,
50 nmol/L EGF, or both 50 nmol/L EGF and 20 nmol/L prexasertib were examined after 48 h of exposure. AKT: protein kinase B; EGF:
epidermal growth factor; EGFR: epidermal growth factor receptor
implanted with MDA-231 or MDA-468. Mice were dosed with vehicle control, prexasertib alone, erlotinib
alone, or a combination of prexasertib and erlotinib. MDA-231 tumors showed similar sensitivity to
prexasertib, erlotinib, and combination treatment [Figure 7A and B]. MDA-468 tumors showed a significant
3
3
reduction in tumor volume after combination treatment (from 338 ± 245 mm to 145 ± 77.3 mm , P = 0.026,
Figure 7A and B). A non-significant reduction in tumor volume was also observed with prexasertib (208
3
3
± 108 mm ) and erlotinib alone (223 ± 101 mm ). No significant decrease in body weight was observed
during monotherapy or combination therapy in either xenograft model [Supplementary Figure 2].
Immunoblot of the final MD-468 tumors showed a trend for reduced EGFR activity in tumors treated
with both prexasertib and erlotinib [Figure 8]. While the tumors did not show a reduction in AKT
phosphorylation in any of the groups, there was an indication of a reduction in BAD phosphorylation
consistent with in vitro work [Figure 5]. Despite the lack of effect on AKT activity, there was still a clear
reduction of tumor volume and tumor weight [Figure 7].
DISCUSSION
Molecular targeting has significantly enhanced survival for hormone and growth factor positive breast
cancers. TNBC has remained challenging to treat because effective molecular targets have not been
identified. CHK1 inhibitors such as prexasertib have shown efficacy as a monotherapy in difficult to treat
tumor types such as neuroblastoma and high-grade serous ovarian tumors [5,7,9] . CHK1 inhibitors’ efficacy
[14]
as monotherapy in TNBC has been limited by modest clinical responses . Recent studies have described
innate and acquired resistance to prexasertib that could help develop biomarkers to stratify patients for
prexasertib monotherapy treatment.
[6]
When characterizing prexasertib in 2015, King et al. noted that the colorectal cancer cell line HCT116 and
[8]
pancreatic carcinoma PANC-1 showed no sensitivity to prexasertib. In 2019, Lowery et al. demonstrated