Page 45 - Read Online
P. 45
Teng et al. Microstructures 2023;3:2023019 https://dx.doi.org/10.20517/microstructures.2023.07 Page 9 of 29
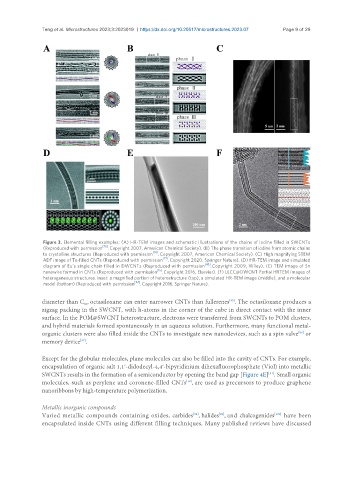
Figure 3. Elemental filling examples: (A) HR-TEM images and schematic illustrations of the chains of iodine filled in SWCNTs
(Reproduced with permission [76] . Copyright 2007, American Chemical Society). (B) The phase transition of iodine from atomic chains
[76]
to crystalline structures (Reproduced with permission . Copyright 2007, American Chemical Society). (C) High magnifying STEM
[77]
ADF image of Te-filled CNTs (Reproduced with permission . Copyright 2020, Springer Nature). (D) HR-TEM image and simulated
[48]
diagram of Eu’s single chain filled in DWCNTs (Reproduced with permission . Copyright 2009, Wiley). (E) TEM image of Sn
[15]
nanowire formed in CNTs (Reproduced with permission . Copyright 2016, Elsevier). (F) LLCC@DWCNT Partial HRTEM images of
heterogeneous structures. Inset: a magnified portion of heterostructure (top), a simulated HR-TEM image (middle), and a molecular
[84]
model (bottom) (Reproduced with permission . Copyright 2016, Springer Nature).
diameter than C , octasiloxane can enter narrower CNTs than fullerenes . The octasiloxane produces a
[95]
60
zigzag packing in the SWCNT, with h-atoms in the corner of the cube in direct contact with the inner
surface. In the POM@SWCNT heterostructure, electrons were transferred from SWCNTs to POM clusters,
and hybrid materials formed spontaneously in an aqueous solution. Furthermore, many functional metal-
organic clusters were also filled inside the CNTs to investigate new nanodevices, such as a spin valve or
[96]
memory device .
[97]
Except for the globular molecules, plane molecules can also be filled into the cavity of CNTs. For example,
encapsulation of organic salt 1,1′-didodecyl-4,4′-bipyridinium dihexafluorophosphate (Viol) into metallic
SWCNTs results in the formation of a semiconductor by opening the band gap [Figure 4E] . Small organic
[21]
molecules, such as perylene and coronene-filled CNTs , are used as precursors to produce graphene
[16]
nanoribbons by high-temperature polymerization.
Metallic inorganic compounds
[100]
Varied metallic compounds containing oxides, carbides , halides , and chalcogenides have been
[98]
[99]
encapsulated inside CNTs using different filling techniques. Many published reviews have discussed