Page 88 - Read Online
P. 88
Yang et al. Microstructures 2023;3:2023013 https://dx.doi.org/10.20517/microstructures.2022.30 Page 21 of 27
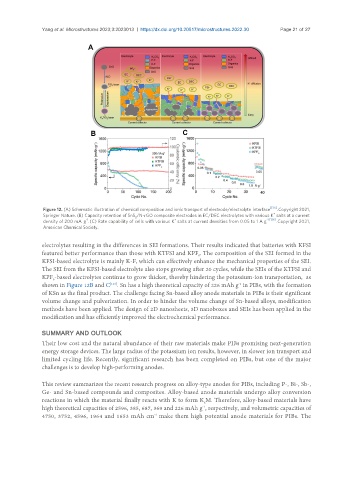
Figure 12. (A) Schematic illustration of chemical composition and ionic transport of electrode/electrolyte interface [135] . Copyright 2021,
+
Springer Nature. (B) Capacity retention of SnS /N-rGO composite electrodes in EC/DEC electrolytes with various K salts at a current
2
-1
+
density of 200 mA g . (C) Rate capability of cells with various K salts at current densities from 0.05 to 1 A g -1 [136] . Copyright 2021,
American Chemical Society.
electrolytes resulting in the differences in SEI formations. Their results indicated that batteries with KFSI
featured better performance than those with KTFSI and KPF . The composition of the SEI formed in the
6
KFSI-based electrolyte is mainly K-F, which can effectively enhance the mechanical properties of the SEI.
The SEI from the KFSI-based electrolyte also stops growing after 20 cycles, while the SEIs of the KTFSI and
KPF -based electrolytes continue to grow thicker, thereby hindering the potassium-ion transportation, as
6
shown in Figure 12B and C . Sn has a high theoretical capacity of 226 mAh g in PIBs, with the formation
-1
[136]
of KSn as the final product. The challenge facing Sn-based alloy anode materials in PIBs is their significant
volume change and pulverization. In order to hinder the volume change of Sn-based alloys, modification
methods have been applied. The design of 2D nanosheets, 3D nanoboxes and SEIs has been applied in the
modification and has efficiently improved the electrochemical performance.
SUMMARY AND OUTLOOK
Their low cost and the natural abundance of their raw materials make PIBs promising next-generation
energy storage devices. The large radius of the potassium ion results, however, in slower ion transport and
limited cycling life. Recently, significant research has been completed on PIBs, but one of the major
challenges is to develop high-performing anodes.
This review summarizes the recent research progress on alloy-type anodes for PIBs, including P-, Bi-, Sb-,
Ge- and Sn-based compounds and composites. Alloy-based anode materials undergo alloy conversion
reactions in which the material finally reacts with K to form K M. Therefore, alloy-based materials have
x
high theoretical capacities of 2596, 385, 687, 369 and 226 mAh g , respectively, and volumetric capacities of
-1
-3
4750, 3752, 4596, 1964 and 1653 mAh cm make them high potential anode materials for PIBs. The