Page 393 - Read Online
P. 393
Iqbal et al. Vessel Plus 2019;3:40 I http://dx.doi.org/10.20517/2574-1209.2019.28 Page 7 of 13
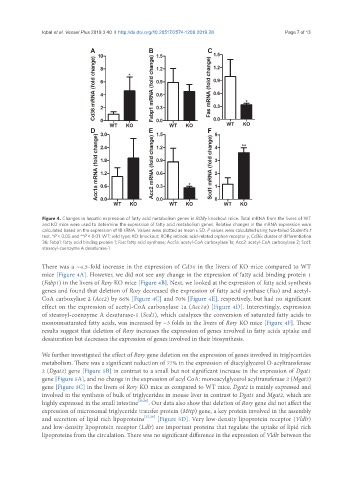
Figure 4. Changes in hepatic expression of fatty acid metabolism genes in RORγ knockout mice. Total mRNA from the livers of WT
and KO mice were used to determine the expression of fatty acid metabolism genes. Relative changes in the mRNA expression were
calculated based on the expression of 18 sRNA. Values were plotted as mean ± SD. P values were calculated using two-tailed Student’s t
test. *P < 0.05 and **P < 0.01. WT: wild type; KO: knockout; RORγ: retinoic acid-related orphan receptor γ; Cd36: cluster of differentiation
36; Fabp1: fatty acid binding protein 1; Fas: fatty acid synthase; Acc1a: acetyl-CoA carboxylase 1a; Acc2: acetyl-CoA carboxylase 2; Scd1:
stearoyl-coenzyme A desaturase-1
There was a ~4.5-fold increase in the expression of Cd36 in the livers of KO mice compared to WT
mice [Figure 4A]. However, we did not see any change in the expression of fatty acid binding protein 1
(Fabp1) in the livers of Rorγ KO mice [Figure 4B]. Next, we looked at the expression of fatty acid synthesis
genes and found that deletion of Rorγ decreased the expression of fatty acid synthase (Fas) and acetyl-
CoA carboxylase 2 (Acc2) by 66% [Figure 4C] and 70% [Figure 4E], respectively, but had no significant
effect on the expression of acetyl-CoA carboxylase 1a (Acc1a) [Figure 4D]. Interestingly, expression
of stearoyl-coenzyme A desaturase-1 (Scd1), which catalyzes the conversion of saturated fatty acids to
monounsaturated fatty acids, was increased by ~3 folds in the livers of Rorγ KO mice [Figure 4F]. These
results suggest that deletion of Rorγ increases the expression of genes involved in fatty acids uptake and
desaturation but decreases the expression of genes involved in their biosynthesis.
We further investigated the effect of Rorγ gene deletion on the expression of genes involved in triglycerides
metabolism. There was a significant reduction of 77% in the expression of diacylglycerol O-acyltransferase
2 (Dgat2) gene [Figure 5B] in contrast to a small but not significant increase in the expression of Dgat1
gene [Figure 5A], and no change in the expression of acyl CoA: monoacylglycerol acyltransferase 2 (Mgat2)
gene [Figure 5C] in the livers of Rorγ KO mice as compared to WT mice. Dgat2 is mainly expressed and
involved in the synthesis of bulk of triglycerides in mouse liver in contrast to Dgat1 and Mgat2, which are
highly expressed in the small intestine [25,26] . Our data also show that deletion of Rorγ gene did not affect the
expression of microsomal triglyceride transfer protein (Mttp) gene, a key protein involved in the assembly
and secretion of lipid rich lipoproteins [27,28] [Figure 5D]. Very low-density lipoprotein receptor (Vldlr)
and low-density lipoprotein receptor (Ldlr) are important proteins that regulate the uptake of lipid rich
lipoproteins from the circulation. There was no significant difference in the expression of Vldlr between the