Page 390 - Read Online
P. 390
Page 4 of 13 Iqbal et al. Vessel Plus 2019;3:40 I http://dx.doi.org/10.20517/2574-1209.2019.28
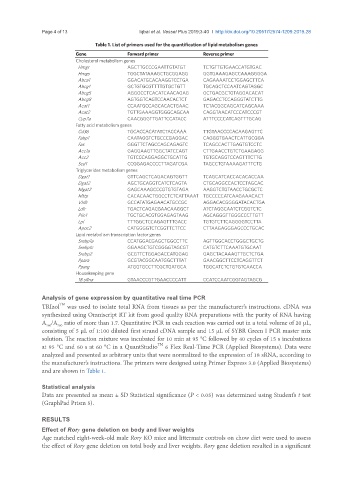
Table 1. List of primers used for the quantification of lipid metabolism genes
Gene Forward primer Reverse primer
Cholesterol metabolism genes
Hmgr AGCTTGCCCGAATTGTATGT TCTGTTGTGAACCATGTGAC
Hmgs TGGCTATAAAGCTGCGGAGG GGTGAAAGAGCCAAAGGGGA
Abca1 GGACATGCACAAGGTCCTGA CAGAAAATCCTGGAGCTTCA
Abcg1 GCTGTGCGTTTTGTGCTGTT TGCAGCTCCAATCAGTAGGC
Abcg5 AGGGCCTCACATCAACAGAG GCTGACGCTGTAGGACACAT
Abcg8 AGTGGTCAGTCCAACACTCT GAGACCTCCAGGGTATCTTG
Acat1 CCAATGCCAGCACACTGAAC TCTACGGCAGCATCAGCAAA
Acat2 TGTTGAAAGGTGGGCAGCAA CAGGTAACATCCCATCCCGT
Cyp7a CAACGGGTTGATTCCATACC ATTTCCCCATCAGTTTGCAG
Fatty acid metabolism genes
Cd36 TGCACCACATATCTACCAAA TTGTAACCCCACAAGAGTTC
Fabp1 CAATAGGTCTGCCCGAGGAC CAGGGTGAACTCATTGCGGA
Fas GGGTTCTAGCCAGCAGAGTC TCAGCCACTTGAGTGTCCTC
Acc1a GAGGAAGTTGGCTATCCAGT CTTGAACCTGTCTGAAGAGG
Acc2 TGTCCCAGGAGGCTGCATTG TGTGCAGGTCCAGTTTCTTG
Scd1 CCGGAGACCCCTTAGATCGA TAGCCTGTAAAAGATTTCTG
Triglycerides metabolism genes
Dgat1 GTTCAGCTCAGACAGTGGTT TCAGCATCACCACACACCAA
Dgat2 AGCTGCAGGTCATCTCAGTA CTGCAGGCCACTCCTAGCAC
Mgat2 GAGCAAAGCCCGTGTGTAGA AAGGTCTGTAACCTGCGCTC
Mttp CACACAACTGGCCTCTCATTAAAT TGCCCCCATCAAGAAACACT
Vldlr GCCATATGAGAACATGCCGC AGGACACGGGGATACACTGA
Ldlr TGACTCAGACGAACAAGGCT ATCTAGGCAATCTCGGTCTC
Plin1 TGCTGCACGTGGAGAGTAAG AGCAGGGTTGGGCCCTTGTT
Lpl TTTGGCTCCAGAGTTTGACC TGTGTCTTCAGGGGTCCTTA
Apoc2 CATGGGGTCTCGGTTCTTCC CTTAAGAGGGAGCCCTGCAC
Lipid metabolism transcription factor genes
Srebp1a CCATGGACGAGCTGGCCTTC AGTTGGCACCTGGGCTGCTG
Srebp1c GGAAGCTGTCGGGGTAGCGT CATGTCTTCAAATGTGCAAT
Srebp2 GCGTTCTGGAGACCATGGAG GAGCTACAAAGTTGCTCTGA
Ppara GCGTACGGCAATGGCTTTAT GAACGGCTTCCTCAGGTTCT
Pparg ATGGTGCCTTCGCTGATGCA TGGCATCTCTGTGTCAACCA
Housekeeping gene
18 sRna GTAACCCGTTGAACCCCATT CCATCCAATCGGTAGTAGCG
Analysis of gene expression by quantitative real time PCR
TM
TRIzol was used to isolate total RNA from tissues as per the manufacturer’s instructions. cDNA was
synthesized using Omniscript RT kit from good quality RNA preparations with the purity of RNA having
A /A ratio of more than 1.7. Quantitative PCR in each reaction was carried out in a total volume of 20 µL,
280
260
consisting of 5 µL of 1:100 diluted first strand cDNA sample and 15 µL of SYBR Green I PCR master mix
solution. The reaction mixture was incubated for 10 min at 95 °C followed by 40 cycles of 15 s incubations
TM
at 95 °C and 60 s at 60 °C in a QuantStudio 6 Flex Real-Time PCR (Applied Biosystems). Data were
analyzed and presented as arbitrary units that were normalized to the expression of 18 sRNA, according to
the manufacturer’s instructions. The primers were designed using Primer Express 3.0 (Applied Biosystems)
and are shown in Table 1.
Statistical analysis
Data are presented as mean ± SD Statistical significance (P < 0.05) was determined using Student’s t test
(GraphPad Prism 5).
RESULTS
Effect of Rorγ gene deletion on body and liver weights
Age matched eight-week-old male Rorγ KO mice and littermate controls on chow diet were used to assess
the effect of Rorγ gene deletion on total body and liver weights. Rorγ gene deletion resulted in a significant