Page 107 - Read Online
P. 107
Page 4 of 11 Flattery et al. Vessel Plus 2024;8:26 https://dx.doi.org/10.20517/2574-1209.2023.130
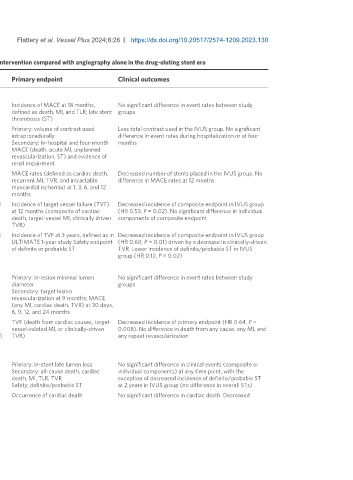
Table 2. Randomized controlled trials of intravascular ultrasound-guided percutaneous coronary intervention compared with angiography alone in the drug-eluting stent era
Name or Year of Design Size Primary endpoint Clinical outcomes
first author publication
Unselected patients
HOME-DES- 2010 Single center. 1:1 randomization of clinically unselected meeting 210 Incidence of MACE at 18 months, No significant difference in event rates between study
IVUS insurance criteria for intravascular ultrasound (IVUS) (complex defined as death, MI, and TLR; late stent groups
coronary lesions or patient characteristics) thrombosis (ST)
MOZART 2014 Single center. Block randomization of clinically unselected patients 83 Primary: volume of contrast used Less total contrast used in the IVUS group. No significant
scheduled for PCI with known risk of contrast-induced acute kidney intraprocedurally difference in event rates during hospitalization or at four
injury. Guidelines provided to reduce contrast use in both arms with Secondary: In-hospital and four-month months
further specific guidance for reducing contrast with IVUS MACE (death, acute MI, unplanned
revascularization, ST) and evidence of
renal impairment
Wang 2015 Single center. 1:1 consecutive randomization of patients with ST- 80 MACE rates (defined as cardiac death, Decreased number of stents placed in the IVUS group. No
elevation MI and high angiographic thrombus burden. IVUS group recurrent MI, TVR, and intractable difference in MACE rates at 12 months
divided into low/high-risk based on IVUS findings with prespecified myocardial ischemia) at 1, 3, 6, and 12
criteria to decrease DES implantation in low risk months
ULTIMATE 2018 Multicenter. 1:1 randomization of clinically unselected patients with 1,448 Incidence of target vessel failure (TVF) Decreased incidence of composite endpoint in IVUS group
(1-year) de novo coronary lesions. Chronic total occlusions, severe at 12 months (composite of cardiac (HR 0.53, P = 0.02). No significant difference in individual
calcification, and inexperienced operators excluded death, target-vessel MI, clinically driven components of composite endpoint
TVR)
ULTIMATE 2021 Multicenter. 1:1 randomization of clinically unselected patients with 1,448 Incidence of TVF at 3 years, defined as in Decreased incidence of composite endpoint in IVUS group
(3-year) de novo coronary lesions. Chronic total occlusions, severe ULTIMATE 1-year study Safety endpoint (HR 0.60, P = 0.01) driven by a decrease in clinically-driven
calcification, and inexperienced operators excluded of definite or probable ST TVR. Lower incidence of definite/probable ST in IVUS
group (HR 0.12, P = 0.02)
Complex lesions
AVIO 2013 Multicenter. 1:1 randomization of patients with stable coronary 284 Primary: in-lesion minimal lumen No significant difference in event rates between study
disease or unstable angina with complex lesions (> 28 mm, CTO, diameter groups
bifurcation, < 2.5mm, four or more stents required) Secondary: target lesion
revascularization at 9 months; MACE
(any MI, cardiac death, TVR) at 30 days,
6, 9, 12, and 24 months
RENOVATE 2023 Multicenter. 2:1 randomization of clinically unselected patients with 1,639 TVF (death from cardiac causes, target- Decreased incidence of primary endpoint (HR 0.64, P =
complex coronary disease (bifurcation, CTO, unprotected left main, (800 vessel-related MI, or clinically-driven 0.008). No difference in death from any cause, any MI, and
long lesions (> 38 mm stent), multivessel PCI, three+ stents, in- IVUS) TVR) any repeat revascularization
stent restenosis, severely calcified, ostial lesions)
Chronic total occlusions
AIR-CTO 2015 Multicenter. 1:1 randomization. Clinically unselected patients with at 230 Primary: in-stent late lumen loss No significant difference in clinical events (composite or
least one CTO randomized after initial lesion crossing to IVUS Secondary: all-cause death, cardiac individual components) at any time point, with the
optimization or angiography alone death, MI, TLR, TVR exception of decreased incidence of definite/probable ST
Safety: definite/probable ST at 2 years in IVUS group (no difference in overall STs)
CTO-IVUS 2015 Multicenter. 1:1 randomization stratified by center. Patients with 402 Occurrence of cardiac death No significant difference in cardiac death. Decreased