Page 91 - Read Online
P. 91
Calafiore et al. Vessel Plus 2023;7:18 https://dx.doi.org/10.20517/2574-1209.2023.42 Page 5 of 21
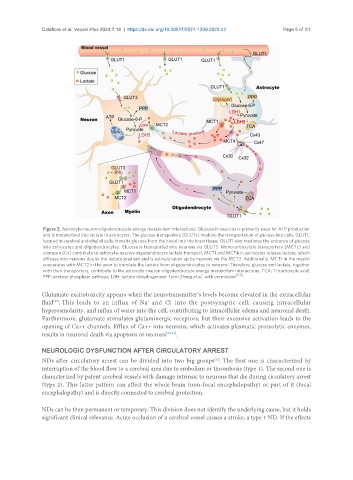
Figure 2. Astrocyte-neuron-oligodendrocyte energy metabolism interactions. Glucose in neurons is primarily used for ATP production
and is metabolized into lactate in astrocytes. The glucose transporters (GLUTs) mediate the transportation of glucose into cells. GLUT1,
located in cerebral endothelial cells, transits glucose from the blood into the brain tissue. GLUT1 also mediates the entrance of glucose
into astrocytes and oligodendrocytes. Glucose is transported into neurons via GLUT3. Monocarboxylate transporters (MCTs) and
connexin (Cx) contribute to astrocyte-neuron-oligodendrocyte lactate transport. MCT1 and MCT4 in astrocytes release lactate, which
diffuses into neurons due to the lactate gradient and is actively taken up by neurons via the MCT2. Additionally, MCT1 in the myelin
cooperates with MCT2 in the axon to translate the lactate from oligodendrocytes to neurons. Therefore, glucose and lactate, together
with their transporters, contribute to the astrocyte-neuron-oligodendrocyte energy metabolism interactions. TCA: Tricarboxylic acid;
[128]
PPP: pentose phosphate pathway; LDH: lactate dehydrogenase. From Zhang et al., with permission .
Glutamate excitotoxicity appears when the neurotransmitter’s levels become elevated in the extracellular
-
fluid . This leads to an influx of Na and Cl into the postsynaptic cell, causing intracellular
[28]
+
hyperosmolarity, and influx of water into the cell, contributing to intracellular edema and neuronal death.
Furthermore, glutamate stimulates glutaminergic receptors, but their excessive activation leads to the
opening of Ca++ channels. Efflux of Ca++ into neurons, which activates plasmatic proteolytic enzymes,
results in neuronal death via apoptosis or necrosis [29-31] .
NEUROLOGIC DYSFUNCTION AFTER CIRCULATORY ARREST
NDs after circulatory arrest can be divided into two big groups . The first one is characterized by
[32]
interruption of the blood flow to a cerebral area due to embolism or thrombosis (type 1). The second one is
characterized by patent cerebral vessels with damage intrinsic to neurons that die during circulatory arrest
(type 2). This latter pattern can affect the whole brain (non-focal encephalopathy) or part of it (focal
encephalopathy) and is directly connected to cerebral protection.
NDs can be then permanent or temporary. This division does not identify the underlying cause, but it holds
significant clinical relevance. Acute occlusion of a cerebral vessel causes a stroke, a type 1 ND. If the effects