Page 90 - Read Online
P. 90
Page 4 of 21 Calafiore et al. Vessel Plus 2023;7:18 https://dx.doi.org/10.20517/2574-1209.2023.42
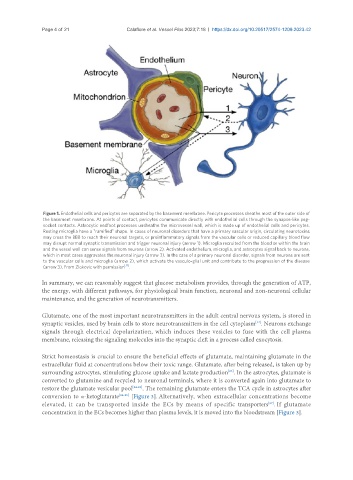
Figure 1. Endothelial cells and pericytes are separated by the basement membrane. Pericyte processes sheathe most of the outer side of
the basement membrane. At points of contact, pericytes communicate directly with endothelial cells through the synapse-like peg-
socket contacts. Astrocytic endfoot processes unsheathe the microvessel wall, which is made up of endothelial cells and pericytes.
Resting microglia have a "ramified" shape. In cases of neuronal disorders that have a primary vascular origin, circulating neurotoxins
may cross the BBB to reach their neuronal targets, or proinflammatory signals from the vascular cells or reduced capillary blood flow
may disrupt normal synaptic transmission and trigger neuronal injury (arrow 1). Microglia recruited from the blood or within the brain
and the vessel wall can sense signals from neurons (arrow 2). Activated endothelium, microglia, and astrocytes signal back to neurons,
which in most cases aggravates the neuronal injury (arrow 3). In the case of a primary neuronal disorder, signals from neurons are sent
to the vascular cells and microglia (arrow 2), which activate the vasculo-glial unit and contribute to the progression of the disease
[11]
(arrow 3). From Zlokovic with permission .
In summary, we can reasonably suggest that glucose metabolism provides, through the generation of ATP,
the energy, with different pathways, for physiological brain function, neuronal and non-neuronal cellular
maintenance, and the generation of neurotransmitters.
Glutamate, one of the most important neurotransmitters in the adult central nervous system, is stored in
synaptic vesicles, used by brain cells to store neurotransmitters in the cell cytoplasm . Neurons exchange
[22]
signals through electrical depolarization, which induces these vesicles to fuse with the cell plasma
membrane, releasing the signaling molecules into the synaptic cleft in a process called exocytosis.
Strict homeostasis is crucial to ensure the beneficial effects of glutamate, maintaining glutamate in the
extracellular fluid at concentrations below their toxic range. Glutamate, after being released, is taken up by
surrounding astrocytes, stimulating glucose uptake and lactate production . In the astrocytes, glutamate is
[23]
converted to glutamine and recycled to neuronal terminals, where it is converted again into glutamate to
restore the glutamate vesicular pool [24-26] . The remaining glutamate enters the TCA cycle in astrocytes after
conversion to α-ketoglutarate [24-26] [Figure 3]. Alternatively, when extracellular concentrations become
[27]
elevated, it can be transported inside the ECs by means of specific transporters . If glutamate
concentration in the ECs becomes higher than plasma levels, it is moved into the bloodstream [Figure 3].