Page 39 - Read Online
P. 39
Park et al. Soft Sci 2024;4:28 https://dx.doi.org/10.20517/ss.2024.22 Page 13 of 28
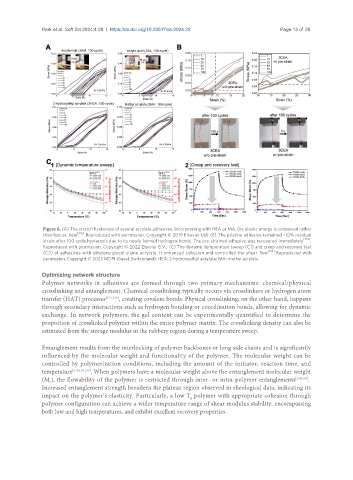
Figure 6. (A) The stretch hysteresis of several acrylate adhesives. Incorporating with HEA or MA, the elastic energy is conserved rather
than lost as heat [126] . Reproduced with permission. Copyright © 2019 Elsevier Ltd; (B) The pristine adhesive remained ~10% residual
strain after 100 cycle hysteresis due to its newly formed hydrogen bonds. The pre-strained adhesive was recovered immediately [128] .
Reproduced with permission. Copyright © 2022 Elsevier B.V.; (C) The dynamic temperature sweep (C1) and creep and recovery test
(C2) of adhesives with ethylene glycol silane acrylate. It enhanced cohesion and controlled the shear flow [132] . Reproduced with
permission. Copyright © 2023 MDPI (Basel, Switzerland). HEA: 2-hydroxyethyl acrylate; MA: methyl acrylate.
Optimizing network structure
Polymer networks in adhesives are formed through two primary mechanisms: chemical/physical
crosslinking and entanglement. Chemical crosslinking typically occurs via crosslinkers or hydrogen atom
transfer (HAT) processes [133-135] , creating covalent bonds. Physical crosslinking, on the other hand, happens
through secondary interactions such as hydrogen bonding or coordination bonds, allowing for dynamic
exchange. In network polymers, the gel content can be experimentally quantified to determine the
proportion of crosslinked polymer within the entire polymer matrix. The crosslinking density can also be
estimated from the storage modulus in the rubbery region during a temperature sweep.
Entanglement results from the interlocking of polymer backbones or long side chains and is significantly
influenced by the molecular weight and functionality of the polymer. The molecular weight can be
controlled by polymerization conditions, including the amount of the initiator, reaction time, and
temperature [130,136,137] . When polymers have a molecular weight above the entanglement molecular weight
(M ), the flowability of the polymer is restricted through inter- or intra-polymer entanglements [138,139] .
e
Increased entanglement strength broadens the plateau region observed in rheological data, indicating its
impact on the polymer’s elasticity. Particularly, a low T polymer with appropriate cohesion through
g
polymer configuration can achieve a wider temperature range of shear modulus stability, encompassing
both low and high temperatures, and exhibit excellent recovery properties.