Page 140 - Read Online
P. 140
Lin et al. Plast Aesthet Res 2019;6:16 I http://dx.doi.org/10.20517/2347-9264.2019.23 Page 3 of 10
A B
C
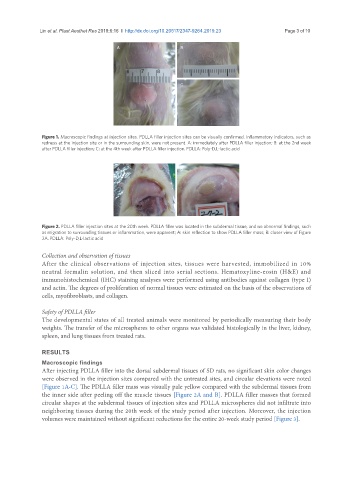
Figure 1. Macroscopic findings at injection sites. PDLLA filler injection sites can be visually confirmed. Inflammatory indicators, such as
redness at the injection site or in the surrounding skin, were not present. A: immediately after PDLLA filler injection; B: at the 2nd week
after PDLLA filler injection; C: at the 4th week after PDLLA filler injection. PDLLA: Poly-D,L-lactic acid
A B
Figure 2. PDLLA filler injection sites at the 20th week. PDLLA filler was located in the subdermal tissue, and no abnormal findings, such
as migration to surrounding tissues or inflammation, were apparent; A: skin reflection to show PDLLA filler mass; B: closer view of Figure
2A. PDLLA: Poly-D,L-lactic acid
Collection and observation of tissues
After the clinical observations of injection sites, tissues were harvested, immobilized in 10%
neutral formalin solution, and then sliced into serial sections. Hematoxyline-eosin (H&E) and
immunohistochemical (IHC) staining analyses were performed using antibodies against collagen (type I)
and actin. The degrees of proliferation of normal tissues were estimated on the basis of the observations of
cells, myofibroblasts, and collagen.
Safety of PDLLA filler
The developmental states of all treated animals were monitored by periodically measuring their body
weights. The transfer of the microspheres to other organs was validated histologically in the liver, kidney,
spleen, and lung tissues from treated rats.
RESULTS
Macroscopic findings
After injecting PDLLA filler into the dorsal subdermal tissues of SD rats, no significant skin color changes
were observed in the injection sites compared with the untreated sites, and circular elevations were noted
[Figure 1A-C]. The PDLLA filler mass was visually pale yellow compared with the subdermal tissues from
the inner side after peeling off the muscle tissues [Figure 2A and B]. PDLLA filler masses that formed
circular shapes at the subdermal tissues of injection sites and PDLLA microspheres did not infiltrate into
neighboring tissues during the 20th week of the study period after injection. Moreover, the injection
volumes were maintained without significant reductions for the entire 20-week study period [Figure 3].