Page 352 - Read Online
P. 352
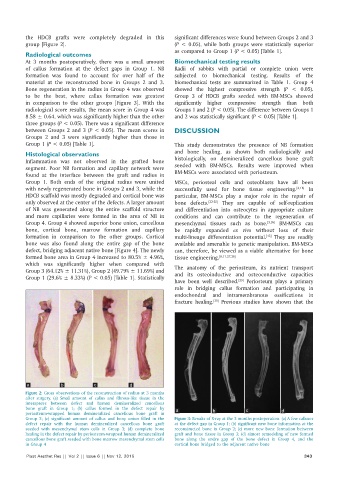
the HDCB grafts were completely degraded in this significant differences were found between Groups 2 and 3
group [Figure 2]. (P < 0.05), while both groups were statistically superior
as compared to Group 1 (P < 0.05) [Table 1].
Radiological outcomes
At 3 months postoperatively, there was a small amount Biomechanical testing results
of callus formation at the defect gaps in Group 1. NB Radii of rabbits with partial or complete union were
formation was found to account for over half of the subjected to biomechanical testing. Results of the
material at the reconstructed bone in Groups 2 and 3. biomechanical tests are summarized in Table 1. Group 4
Bone regeneration in the radius in Group 4 was observed showed the highest compressive strength (P < 0.05).
to be the best, where callus formation was greatest Group 3 of HDCB grafts seeded with BM‑MSCs showed
in comparison to the other groups [Figure 3]. With the significantly higher compressive strength than both
radiological score results, the mean score in Group 4 was Groups 1 and 2 (P < 0.05). The difference between Groups 1
8.58 ± 0.64, which was significantly higher than the other and 2 was statistically significant (P < 0.05) [Table 1].
three groups (P < 0.05). There was a significant difference
between Groups 2 and 3 (P < 0.05). The mean scores in DISCUSSION
Groups 2 and 3 were significantly higher than those in
Group 1 (P < 0.05) [Table 1]. This study demonstrates the presence of NB formation
Histological observations and bone healing, as shown both radiologically and
Inflammation was not observed in the grafted bone histologically, on demineralized cancellous bone graft
segment. Poor NB formation and capillary network were seeded with BM‑MSCs. Results were improved when
found at the interface between the graft and radius in BM‑MSCs were associated with periosteum.
Group 1. Both ends of the original radius were united MSCs, periosteal cells and osteoblasts have all been
with newly regenerated bone in Groups 2 and 3, while the successfully used for bone tissue engineering. [4,18] In
HDCB scaffold was mostly degraded and cortical bone was particular, BM‑MSCs play a major role in the repair of
only observed at the center of the defects. A larger amount bone defects. [22‑25] They are capable of self‑replication
of NB was generated along the entire scaffold structure and differentiation into osteocytes in appropriate culture
and more capillaries were formed in the area of NB in conditions and can contribute to the regeneration of
Group 4. Group 4 showed superior bone union, cancellous mesenchymal tissues such as bone. [3,26] BM‑MSCs can
bone, cortical bone, marrow formation and capillary be rapidly expanded ex vivo without loss of their
formation in comparison to the other groups. Cortical multi‑lineage differentiation potential. They are readily
[13]
bone was also found along the entire gap of the bone available and amenable to genetic manipulation. BM‑MSCs
defect, bridging adjacent native bone [Figure 4]. The newly can, therefore, be viewed as a viable alternative for bone
formed bone area in Group 4 increased to 80.5% ± 4.96%, tissue engineering. [8,11,27,28]
which was significantly higher when compared with The anatomy of the periosteum, its nutrient transport
Group 3 (64.12% ± 11.31%), Group 2 (49.79% ± 11.69%) and and its osteoinductive and osteoconductive capacities
Group 1 (29.6% ± 8.33%) (P < 0.05) [Table 1]. Statistically
have been well described. Periosteum plays a primary
[29]
role in bridging callus formation and participating in
endochondral and intramembranous ossifications in
fracture healing. Previous studies have shown that the
[30]
a b c d
Figure 2: Gross observations of the reconstruction of radius at 3 months
after surgery. (a) Small amount of callus and fibrous‑like tissue in the
interspaces between defect and human demineralized cancellous
bone graft in Group 1; (b) callus formed in the defect repair by a b c d
periosteum‑wrapped human demineralized cancellous bone graft in
Group 2; (c) significant amount of callus and bony union filled in the Figure 3: Results of X‑ray at the 3 months postoperation. (a) A few calluses
defect repair with the human demineralized cancellous bone graft at the defect gap in Group 1; (b) significant new bone information at the
seeded with mesenchymal stem cells in Group 3; (d) complete bone reconstructed bone in Group 2; (c) more new bone formation between
healing in the defect repair by periosteum‑wrapped human demineralized graft and bone tissue in Group 3; (d) almost remodeling of new formed
cancellous bone graft seeded with bone marrow mesenchymal stem cells bone along the entire gap of the bone defect in Group 4, and the
in Group 4 cortical bone bridged to the adjacent native bone
Plast Aesthet Res || Vol 2 || Issue 6 || Nov 12, 2015 343