Page 86 - Read Online
P. 86
Table 3: Cellular skin substitutes for diabetic chronic ulcers (costs at the time of writing should be treated
as a guide and have been converted into € currency where necessary; the name of the scaffold is present if the
product has no commercial name)
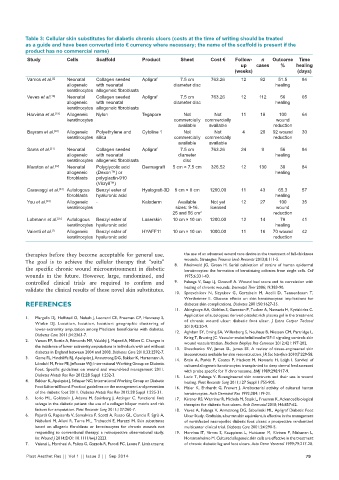
Study Cells Scaffold Product Sheet Cost € Follow- n Outcome Time
up cases % healing
(weeks) (days)
Vamos et al. [2] Neonatal Collagen seeded Apligraf 7.5 cm 763.26 12 82 51.5 84
allogeneic with neonatal diameter disc healing
keratinocytes allogeneic fibroblasts
Veves et al. [18] Neonatal Collagen seeded Apligraf 7.5 cm 763.26 12 112 56 65
allogeneic with neonatal diameter disc healing
keratinocytes allogeneic fibroblasts
Harvima et al. [19] Allogeneic Nylon Tegapore Not Not 11 18 100 64
keratinocytes commercially commercially wound
available available reduction
Bayram et al. [20] Allogeneic Polyethylene and Cytoline 1 Not Not 4 20 92 wound 30
keratinocytes silica commercially commercially reduction
available available
Sams et al. [21] Neonatal Collagen seeded Apligraf 7.5 cm 763.26 24 9 56 84
allogeneic with neonatal diameter healing
keratinocytes allogeneic fibroblasts disc
Marston et al. [22] Neonatal Polyglycolic acid Dermagraft 5 cm × 7.5 cm 326.52 12 130 30 84
allogeneic (Dexon™) or healing
fibroblasts polyglactin-910
(Vicryil™)
Caravaggi et al. [23] Autologous Benzyl ester of Hyalograft-3D 8 cm × 8 cm 1200.00 11 43 65.3 57
fibroblasts hyaluronic acid healing
You et al. [24] Allogeneic - Kaloderm Available Not yet 12 27 100 35
keratinocytes sizes: 9-16. licensed wound
25 and 56 cm 2 reduction
Lobmann et al. [25] Autologous Benzyl ester of Laserskin 10 cm × 10 cm 1200.00 12 14 78 41
keratinocytes hyaluronic acid healing
Vaienti et al. [7] Allogeneic Benzyl ester of HYAFF11 10 cm × 10 cm 1000.00 11 16 70 wound 42
keratinocytes hyaluronic acid reduction
therapies before they become acceptable for general use. the use of an advanced wound care device in the treatment of full‑thickness
The goal is to achieve the cellular therapy that “suits” wounds. Strategies Trauma Limb Reconstr 2013;8:111‑5.
the specific chronic wound microenvironment in diabetic 8. Rheinwald JG, Green H. Serial cultivation of strains of human epidermal
keratinocytes: the formation of keratinizing colonies from single cells. Cell
wounds in the future. However, large, randomized, and 1975;6:331‑43.
controlled clinical trials are required to confirm and 9. Falanga V, Saap LJ, Ozonoff A. Wound bed score and its correlation with
validate the clinical results of these novel skin substitutes. healing of chronic wounds. Dermatol Ther 2006;19:383‑90.
10. Spravchikov N, Sizyakov G, Gartsbein M, Accili D, Tennenbaum T,
Wertheimer E. Glucose effects on skin keratinocytes: implications for
REFERENCES diabetes skin complications. Diabetes 2001;50:1627‑35.
11. Akingboye AA, Giddins S, Gamston P, Tucker A, Navsaria H, Kyriakides C.
Application of autologous derived‑platelet rich plasma gel in the treatment
1. Margolis DJ, Hoffstad O, Nafash J, Leonard CE, Freeman CP, Hennessy S, of chronic wound ulcer: diabetic foot ulcer. J Extra Corpor Technol
Wiebe DJ. Location, location, location: geographic clustering of 2010;42:20‑9.
lower‑extremity amputation among Medicare beneficiaries with diabetes. 12. Aghdam SY, Eming SA, Willenborg S, Neuhaus B, Niessen CM, Partridge L,
Diabetes Care 2011;34:2363‑7. Krieg T, Bruning JC. Vascular endothelial insulin/GF‑1 signaling controls skin
2. Vamos EP, Bottle A, Edmonds ME, Valabhji J, Majeed A, Millett C. Changes in wound vascularization. Biochem Biophys Res Commun 2012;421:197‑202.
the incidence of lower extremity amputations in individuals with and without 13. Shevchenko RV, James SL, James SE. A review of tissue‑engineered skin
diabetes in England between 2004 and 2008. Diabetes Care 2010;33:2592‑7. bioconstructs available for skin reconstruction. J R Soc Interface 2010;7:229‑58.
3. Game FL, Hinchliffe RJ, Apelqvist J, Armstrong DG, Bakker K, Hartemann A, 14. Brain A, Purkis P, Coates P, Hackett M, Navsaria H, Leigh I. Survival of
Löndahl M, Price PE, Jeffcoate WJ; International Working Group on Diabetic cultured allogeneic keratinocytes transplanted to deep dermal bed assessed
Foot. Specific guidelines on wound and wound‑bed management 2011. with probe specific for Y chromosome. BMJ 1989;298:917‑9.
Diabetes Metab Res Rev 2012;28 Suppl 1:232‑3. 15. Lazic T, Falanga V. Bioengineered skin constructs and their use in wound
4. Bakker K, Apelqvist J, Schaper NC; International Working Group on Diabetic healing. Plast Reconstr Surg 2011;127 Suppl 1:75S‑90S.
Foot Editorial Board. Practical guidelines on the management and prevention 16. Maier K, Ehrhardt G, Frevert J. Antibacterial activity of cultured human
of the diabetic foot 2011. Diabetes Metab Res Rev 2012;28 Suppl 1:225‑31. keratinocytes. Arch Dermatol Res 1992;284:119‑21.
5. Iorio ML, Goldstein J, Adams M, Steinberg J, Attinger C. Functional limb 17. Kirsner RS, Warriner R, Michela M, Stasik L, Freeman K. Advanced biological
salvage in the diabetic patient: the use of a collagen bilayer matrix and risk therapies for diabetic foot ulcers. Arch Dermatol 2010;146:857‑62.
factors for amputation. Plast Reconstr Surg 2011;127:260‑7. 18. Veves A, Falanga V, Armstrong DG, Sabolinski ML, Apligraf Diabetic Foot
6. Pajardi G, Rapisarda V, Somalvico F, Scotti A, Russo GL, Ciancio F, Sgrò A, Ulcer Study. Graftskin, a human skin equivalent, is effective in the management
Nebuloni M, Allevi R, Torre ML, Trabucchi E, Marazzi M. Skin substitutes of noninfected neuropathic diabetic foot ulcers: a prospective randomized
based on allogenic fibroblasts or keratinocytes for chronic wounds not multicenter clinical trial. Diabetes Care 2001;24:290‑5.
responding to conventional therapy: a retrospective observational study. 19. Harvima IT, Virnes S, Kauppinen L, Huttunen M, Kivinen P, Niskanen L,
Int Wound J 2014;DOI: 10.1111/iwj.12223. Horsmanheimo M. Cultured allogeneic skin cells are effective in the treatment
7. Vaienti L, Marchesi A, Palitta G, Gazzola R, Parodi PC, Leone F. Limb trauma: of chronic diabetic leg and foot ulcers. Acta Derm Venereol 1999;79:217‑20.
Plast Aesthet Res || Vol 1 || Issue 2 || Sep 2014 79