Page 68 - Read Online
P. 68
Tanner et al. Plast Aesthet Res 2023;10:11 https://dx.doi.org/10.20517/2347-9264.2022.95 Page 9 of 12
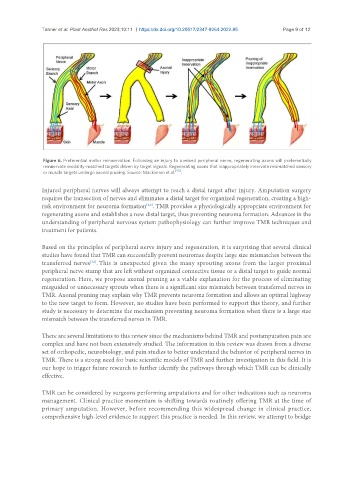
Figure 6. Preferential motor reinnervation. Following an injury to a mixed peripheral nerve, regenerating axons will preferentially
reinnervate modality-matched targets driven by target signals. Regenerating axons that inappropriately innervate mismatched sensory
or muscle targets undergo axonal pruning. Source: Mackinnon et al. [43] .
Injured peripheral nerves will always attempt to reach a distal target after injury. Amputation surgery
requires the transection of nerves and eliminates a distal target for organized regeneration, creating a high-
[4,8]
risk environment for neuroma formation . TMR provides a physiologically appropriate environment for
regenerating axons and establishes a new distal target, thus preventing neuroma formation. Advances in the
understanding of peripheral nervous system pathophysiology can further improve TMR techniques and
treatment for patients.
Based on the principles of peripheral nerve injury and regeneration, it is surprising that several clinical
studies have found that TMR can successfully prevent neuromas despite large size mismatches between the
transferred nerves . This is unexpected given the many sprouting axons from the larger proximal
[4,8]
peripheral nerve stump that are left without organized connective tissue or a distal target to guide normal
regeneration. Here, we propose axonal pruning as a viable explanation for the process of eliminating
misguided or unnecessary sprouts when there is a significant size mismatch between transferred nerves in
TMR. Axonal pruning may explain why TMR prevents neuroma formation and allows an optimal highway
to the new target to form. However, no studies have been performed to support this theory, and further
study is necessary to determine the mechanism preventing neuroma formation when there is a large size
mismatch between the transferred nerves in TMR.
There are several limitations to this review since the mechanisms behind TMR and postamputation pain are
complex and have not been extensively studied. The information in this review was drawn from a diverse
set of orthopedic, neurobiology, and pain studies to better understand the behavior of peripheral nerves in
TMR. There is a strong need for basic scientific models of TMR and further investigation in this field. It is
our hope to trigger future research to further identify the pathways through which TMR can be clinically
effective.
TMR can be considered by surgeons performing amputations and for other indications such as neuroma
management. Clinical practice momentum is shifting towards routinely offering TMR at the time of
primary amputation. However, before recommending this widespread change in clinical practice,
comprehensive high-level evidence to support this practice is needed. In this review, we attempt to bridge