Page 12 - Read Online
P. 12
Reilly et al. Plast Aesthet Res 2021;8:2 I http://dx.doi.org/10.20517/2347-9264.2020.153 Page 5 of 24
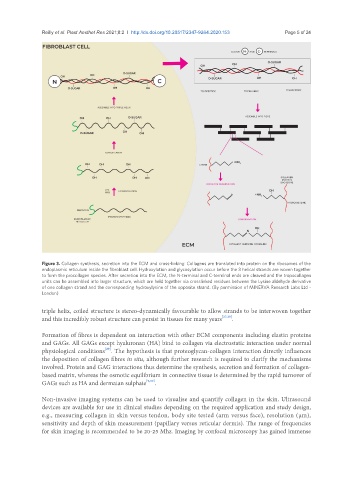
Figure 3. Collagen synthesis, secretion into the ECM and cross-linking. Collagens are translated into protein on the ribosomes of the
endoplasmic reticulum inside the fibroblast cell. Hydroxylation and glycosylation occur before the 3 helical strands are woven together
to form the procollagen species. After secretion into the ECM, the N-terminal and C-terminal ends are cleaved and the tropocollagen
units can be assembled into larger structure, which are held together via crosslinked residues between the Lysine aldehyde derivative
of one collagen strand and the corresponding hydroxylysine of the opposite strand. (By permission of MINERVA Research Labs Ltd -
London)
triple helix, coiled structure is stereo-dynamically favourable to allow strands to be interwoven together
and this incredibly robust structure can persist in tissues for many years [27,28] .
Formation of fibres is dependent on interaction with other ECM components including elastin proteins
and GAGs. All GAGs except hyaluronan (HA) bind to collagen via electrostatic interaction under normal
[29]
physiological conditions . The hypothesis is that proteoglycan-collagen interaction directly influences
the deposition of collagen fibres in situ, although further research is required to clarify the mechanisms
involved. Protein and GAG interactions thus determine the synthesis, secretion and formation of collagen-
based matrix, whereas the osmotic equilibrium in connective tissue is determined by the rapid turnover of
GAGs such as HA and dermatan sulphate [9,30] .
Non-invasive imaging systems can be used to visualise and quantify collagen in the skin. Ultrasound
devices are available for use in clinical studies depending on the required application and study design,
e.g., measuring collagen in skin versus tendon, body site tested (arm versus face), resolution (µm),
sensitivity and depth of skin measurement (papillary versus reticular dermis). The range of frequencies
for skin imaging is recommended to be 20-25 Mhz. Imaging by confocal microscopy has gained immense