Page 10 - Read Online
P. 10
Reilly et al. Plast Aesthet Res 2021;8:2 I http://dx.doi.org/10.20517/2347-9264.2020.153 Page 3 of 24
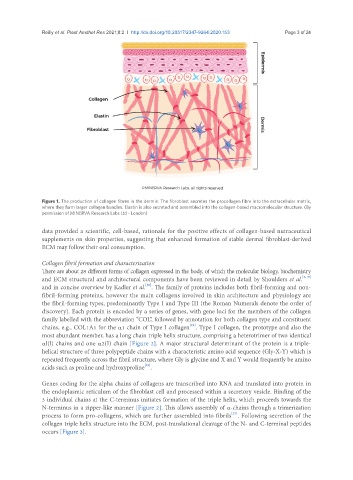
Figure 1. The production of collagen fibres in the dermis. The fibroblast secretes the procollagen fibre into the extracellular matrix,
where they form larger collagen bundles. Elastin is also secreted and assembled into the collagen-based macromolecular structure. (By
permission of MINERVA Research Labs Ltd - London)
data provided a scientific, cell-based, rationale for the positive effects of collagen-based nutraceutical
supplements on skin properties, suggesting that enhanced formation of stable dermal fibroblast-derived
ECM may follow their oral consumption.
Collagen fibril formation and characterization
There are about 28 different forms of collagen expressed in the body, of which the molecular biology, biochemistry
and ECM structural and architectural components have been reviewed in detail by Shoulders et al. [2,19]
[20]
and in concise overview by Kadler et al. . The family of proteins includes both fibril-forming and non-
fibril-forming proteins, however the main collagens involved in skin architecture and physiology are
the fibril-forming types, predominantly Type I and Type III (the Roman Numerals denote the order of
discovery). Each protein is encoded by a series of genes, with gene loci for the members of the collagen
family labelled with the abbreviation “COL”, followed by annotation for both collagen type and constituent
[21]
chains, e.g., COL1A1 for the α1 chain of Type I collagen . Type I collagen, the prototype and also the
most abundant member, has a long chain triple helix structure, comprising a heterotrimer of two identical
αl(l) chains and one α2(I) chain [Figure 2]. A major structural determinant of the protein is a triple-
helical structure of three polypeptide chains with a characteristic amino acid sequence (Gly-X-Y) which is
repeated frequently across the fibril structure, where Gly is glycine and X and Y would frequently be amino
[22]
acids such as proline and hydroxyproline .
Genes coding for the alpha chains of collagens are transcribed into RNA and translated into protein in
the endoplasmic reticulum of the fibroblast cell and processed within a secretory vesicle. Binding of the
3 individual chains at the C-terminus initiates formation of the triple helix, which proceeds towards the
N-terminus in a zipper-like manner [Figure 2]. This allows assembly of α-chains through a trimerization
[23]
process to form pro-collagens, which are further assembled into fibrils . Following secretion of the
collagen triple helix structure into the ECM, post-translational cleavage of the N- and C-terminal peptides
occurs [Figure 3].