Page 21 - Read Online
P. 21
Page 6 of 8 Gavard Molliard et al. Plast Aesthet Res 2018;5:17 I http://dx.doi.org/10.20517/2347-9264.2018.10
Gel with low F N Gel with high F N
Figure 2. Schematic representation of the level of normal force F N for a HA gel. Impact on the shape and behavior of the gel for a product
with a low F N and a high F N
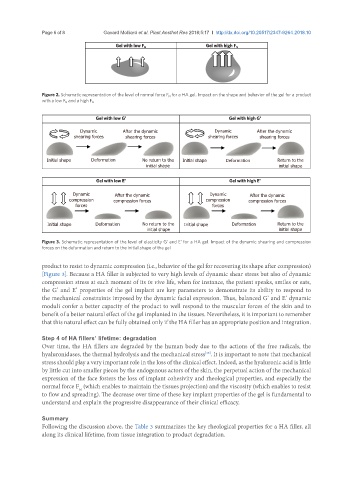
Gel with low G’ Gel with high G’
Dynamic After the dynamic Dynamic After the dynamic
shearing forces shearing forces shearing forces shearing forces
Initial shape Deformation No return to the Initial shape Deformation Return to the
initial shape initial shape
Gel with low E’ Gel with high E’
Dynamic After the dynamic Dynamic After the dynamic
compression compression forces compression compression forces
forces forces
Initial shape Deformation No return to the Initial shape Deformation Return to the
initial shape initial shape
Figure 3. Schematic representation of the level of elasticity G’ and E’ for a HA gel. Impact of the dynamic shearing and compression
forces on the deformation and return to the initial shape of the gel
product to resist to dynamic compression (i.e., behavior of the gel for recovering its shape after compression)
[Figure 3]. Because a HA filler is subjected to very high levels of dynamic shear stress but also of dynamic
compression stress at each moment of its in vivo life, when for instance, the patient speaks, smiles or eats,
the G’ and E’ properties of the gel implant are key parameters to demonstrate its ability to respond to
the mechanical constraints imposed by the dynamic facial expression. Thus, balanced G’ and E’ dynamic
moduli confer a better capacity of the product to well respond to the muscular forces of the skin and to
benefit of a better natural effect of the gel implanted in the tissues. Nevertheless, it is important to remember
that this natural effect can be fully obtained only if the HA filler has an appropriate position and integration.
Step 4 of HA fillers’ lifetime: degradation
Over time, the HA fillers are degraded by the human body due to the actions of the free radicals, the
hyaluronidases, the thermal hydrolysis and the mechanical stress . It is important to note that mechanical
[15]
stress should play a very important role in the loss of the clinical effect. Indeed, as the hyaluronic acid is little
by little cut into smaller pieces by the endogenous actors of the skin, the perpetual action of the mechanical
expression of the face fosters the loss of implant cohesivity and rheological properties, and especially the
normal force F (which enables to maintain the tissues projection) and the viscosity (which enables to resist
N
to flow and spreading). The decrease over time of these key implant properties of the gel is fundamental to
understand and explain the progressive disappearance of their clinical efficacy.
Summary
Following the discussion above, the Table 3 summarizes the key rheological properties for a HA filler, all
along its clinical lifetime, from tissue integration to product degradation.