Page 19 - Read Online
P. 19
Page 4 of 8 Gavard Molliard et al. Plast Aesthet Res 2018;5:17 I http://dx.doi.org/10.20517/2347-9264.2018.10
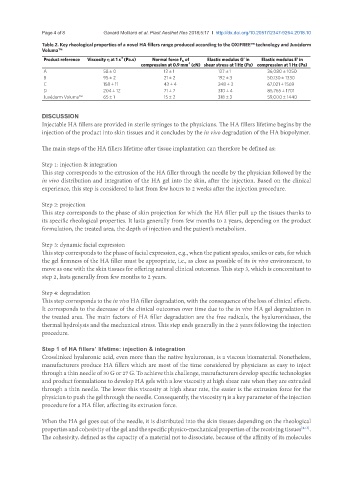
Table 2. Key rheological properties of a novel HA fillers range produced according to the OXIFREE™ technology and Juvéderm
Voluma™
-1
Product reference Viscosity η at 1 s (Pa.s) Normal force F N of Elastic modulus G’ in Elastic modulus E’ in
-1
compression at 0.9 mm (cN) shear stress at 1 Hz (Pa) compression at 1 Hz (Pa)
A 58 ± 0 12 ± 1 137 ± 1 36,080 ± 1050
B 95 ± 2 21 ± 2 192 ± 3 50,130 ± 1330
C 158 ± 11 43 ± 4 248 ± 3 67,021 ± 1569
D 204 ± 12 71 ± 7 310 ± 4 85,765 ± 1701
Juvéderm Voluma™ 65 ± 1 15 ± 2 318 ± 3 59,000 ± 1440
DISCUSSION
Injectable HA fillers are provided in sterile syringes to the physicians. The HA fillers lifetime begins by the
injection of the product into skin tissues and it concludes by the in vivo degradation of the HA biopolymer.
The main steps of the HA fillers lifetime after tissue implantation can therefore be defined as:
Step 1: injection & integration
This step corresponds to the extrusion of the HA filler through the needle by the physician followed by the
in vivo distribution and integration of the HA gel into the skin, after the injection. Based on the clinical
experience, this step is considered to last from few hours to 2 weeks after the injection procedure.
Step 2: projection
This step corresponds to the phase of skin projection for which the HA filler pull up the tissues thanks to
its specific rheological properties. It lasts generally from few months to 2 years, depending on the product
formulation, the treated area, the depth of injection and the patient’s metabolism.
Step 3: dynamic facial expression
This step corresponds to the phase of facial expression, e.g., when the patient speaks, smiles or eats, for which
the gel firmness of the HA filler must be appropriate, i.e., as close as possible of its in vivo environment, to
move as one with the skin tissues for offering natural clinical outcomes. This step 3, which is concomitant to
step 2, lasts generally from few months to 2 years.
Step 4: degradation
This step corresponds to the in vivo HA filler degradation, with the consequence of the loss of clinical effects.
It corresponds to the decrease of the clinical outcomes over time due to the in vivo HA gel degradation in
the treated area. The main factors of HA filler degradation are the free radicals, the hyaluronidases, the
thermal hydrolysis and the mechanical stress. This step ends generally in the 2 years following the injection
procedure.
Step 1 of HA fillers’ lifetime: injection & integration
Crosslinked hyaluronic acid, even more than the native hyaluronan, is a viscous biomaterial. Nonetheless,
manufacturers produce HA fillers which are most of the time considered by physicians as easy to inject
through a thin needle of 30 G or 27 G. To achieve this challenge, manufacturers develop specific technologies
and product formulations to develop HA gels with a low viscosity at high shear rate when they are extruded
through a thin needle. The lower this viscosity at high shear rate, the easier is the extrusion force for the
physician to push the gel through the needle. Consequently, the viscosity η is a key parameter of the injection
procedure for a HA filler, affecting its extrusion force.
When the HA gel goes out of the needle, it is distributed into the skin tissues depending on the rheological
properties and cohesivity of the gel and the specific physico-mechanical properties of the receiving tissues [4,13] .
The cohesivity, defined as the capacity of a material not to dissociate, because of the affinity of its molecules