Page 30 - Read Online
P. 30
Kapaki et al. Neuroimmunol Neuroinflammation 2020;7:319-29 I http://dx.doi.org/10.20517/2347-8659.2019.26 Page 325
Figure 7. Brain magnetic resonance imaging (T1 sequence) of case 5, showing frontal frontoparietal and sylvian atrophy more evident to
the left (A,B) with preservation of the hippocampus (C)
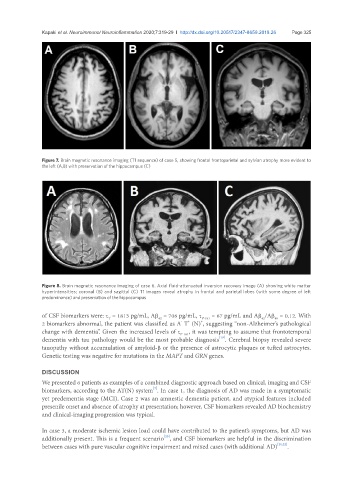
Figure 8. Brain magnetic resonance imaging of case 6. Axial fluid-attenuated inversion recovery image (A) showing white matter
hyperintensities; coronal (B) and sagittal (C) T1 images reveal atrophy in frontal and parietal lobes (with some degree of left
predominance) and preservation of the hippocampus
of CSF biomarkers were: τ = 1813 pg/mL, Aβ = 706 pg/mL, τ P-181 = 67 pg/mL and Aβ /Aβ = 0.12. With
40
42
T
42
-
+
+
2 biomarkers abnormal, the patient was classified as A T (N) , suggesting “non-Alzheimer’s pathological
change with dementia”. Given the increased levels of τ P-181 , it was tempting to assume that frontotemporal
[19]
dementia with tau pathology would be the most probable diagnosis . Cerebral biopsy revealed severe
tauopathy without accumulation of amyloid-β or the presence of astrocytic plaques or tufted astrocytes.
Genetic testing was negative for mutations in the MAPT and GRN genes.
DISCUSSION
We presented 6 patients as examples of a combined diagnostic approach based on clinical, imaging and CSF
[7]
biomarkers, according to the AT(N) system . In case 1, the diagnosis of AD was made in a symptomatic
yet predementia stage (MCI). Case 2 was an amnestic dementia patient, and atypical features included
presenile onset and absence of atrophy at presentation; however, CSF biomarkers revealed AD biochemistry
and clinical-imaging progression was typical.
In case 3, a moderate ischemic lesion load could have contributed to the patient’s symptoms, but AD was
[20]
additionally present. This is a frequent scenario , and CSF biomarkers are helpful in the discrimination
between cases with pure vascular cognitive impairment and mixed cases (with additional AD) [21,22] .