Page 78 - Read Online
P. 78
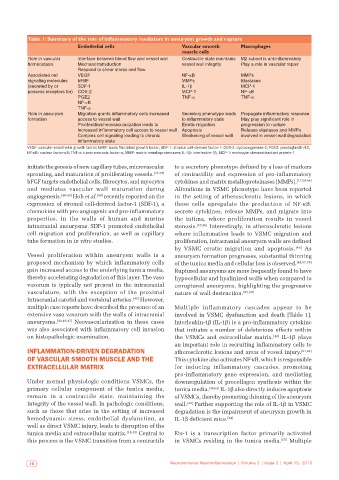
Table 1: Summary of the role of inflammatory mediators in aneurysm growth and rupture
Endothelial cells Vascular smooth Macrophages
muscle cells
Role in vascular Interface between blood flow and vessel wall Contractile state maintains M2 subset is antiinflammatory
homeostasis Mechanotransduction vessel wall integrity Play a role in vascular repair
Respond to shear stress and flow
Associated cell VEGF NF-κB MMPs
signaling molecules bFBF MMPs Elastases
(secreted by or SDF-1 IL-1β MCP-1
possess receptors for) COX-2 MCP-1 NF-κB
PGE2 TNF-α TNF-α
NF-κB
TNF-α
Role in aneurysm Migration grants inflammatory cells increased Secretory phenotype leads Propagate inflammatory response
formation access to vessel wall to inflammatory state May play significant role in
Proliferation/neovascularization leads to Erratic migration progression to rupture
increased inflammatory cell access to vessel wall Apoptosis Release elastases and MMPs
Complex cell signaling leading to chronic Weakening of vessel wall involved in vessel wall degradation
inflammatory state
VEGF: vascular endothelial growth factor; bFBF: basic fibroblast growth factor; SDF‑1: stromal cell‑derived factor‑1; COX‑2: cyclooxygenase‑2; PGE2: prostaglandin E2;
NF‑κB: nuclear factor‑κB; TNF‑α: tumor necrosis factor‑α; MMP: matrix metalloproteinases; IL‑1β: interleukin‑1β; MCP‑1: monocyte chemoattractant protein‑1
initiate the genesis of new capillary tubes, microvascular to a secretory phenotype defined by a loss of markers
sprouting, and maturation of proliferating vessels. [37-39] of contractility and expression of pro-inflammatory
bFGF targets endothelial cells, fibrocytes, and myocytes cytokines and matrix metalloproteinases (MMPs). [17,50-54]
and mediates vascular wall maturation during Alterations in VSMC phenotype have been reported
angiogenesis. [40-43] Hoh et al. [44] recently reported on the in the setting of atherosclerotic lesions, in which
expression of stromal cell-derived factor-1 (SDF-1), a these cells upregulate the production of NF-κB,
chemokine with pro-angiogenic and pro-inflammatory secrete cytokines, release MMPs, and migrate into
properties, in the walls of human and murine the intima, where proliferation results in vessel
intracranial aneurysms. SDF-1 promoted endothelial stenosis. [55,56] Interestingly, in atherosclerotic lesions
cell migration and proliferation, as well as capillary where inflammation leads to VSMC migration and
tube formation in in vitro studies. proliferation, intracranial aneurysm walls are defined
by VSMC erratic migration and apoptosis. [48] As
Vessel proliferation within aneurysm walls is a aneurysm formation progresses, substantial thinning
proposed mechanism by which inflammatory cells of the tunica media and cellular loss is observed. [48,57,58]
gain increased access to the underlying tunica media, Ruptured aneurysms are more frequently found to have
thereby accelerating degradation of this layer. The vaso hypocellular and hyalinized walls when compared to
vasorum is typically not present in the intracranial unruptured aneurysms, highlighting the progressive
vasculature, with the exception of the proximal nature of wall destruction. [59,60]
intracranial carotid and vertebral arteries. [45] However,
multiple case reports have described the presence of an Multiple inflammatory cascades appear to be
extensive vaso vasorum with the walls of intracranial involved in VSMC dysfunction and death [Table 1].
aneurysms. [44,46,47] Neovascularization in these cases Interleukin-1β (IL-1β) is a pro-inflammatory cytokine
was also associated with inflammatory cell invasion that initiates a number of deleterious effects within
on histopathologic examination. the VSMCs and extracellular matrix. [48] IL-1β plays
an important role in recruiting inflammatory cells to
INFLAMMATION‑DRIVEN DEGRADATION atherosclerotic lesions and areas of vessel injury. [61,62]
OF VASCULAR SMOOTH MUSCLE AND THE This cytokine also activates NF-κB, which is responsible
EXTRACELLULAR MATRIX for inducing inflammatory cascades, promoting
pro-inflammatory gene expression, and mediating
Under normal physiologic conditions VSMCs, the downregulation of procollagen synthesis within the
primary cellular component of the tunica media, tunica media. [48,63] IL-1β also directly induces apoptosis
remain in a contractile state, maintaining the of VSMCs, thereby promoting thinning of the aneurysm
integrity of the vessel wall. In pathologic conditions, wall. [48] Further supporting the role of IL-1β in VSMC
such as those that arise in the setting of increased degradation is the impairment of aneurysm growth in
hemodynamic stress, endothelial dysfunction, as IL-1β deficient mice. [64]
well as direct VSMC injury, leads to disruption of the
tunica media and extracellular matrix. [48,49] Central to Ets-1 is a transcription factor primarily activated
this process is the VSMC transition from a contractile in VSMCs residing in the tunica media. [65] Multiple
70 Neuroimmunol Neuroinflammation | Volume 2 | Issue 2 | April 15, 2015