Page 77 - Read Online
P. 77
a b
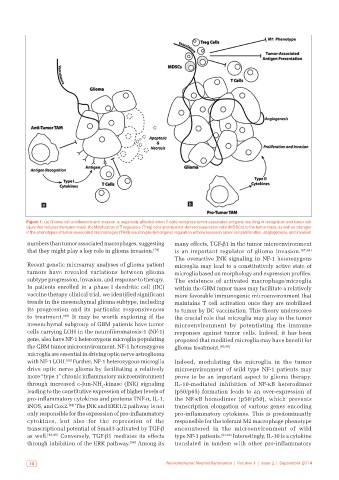
Figure 1: (a) Glioma cell proliferation and invasion is negatively affected when T cells recognize tumor‑associated antigens resulting in recognition and tumor cell
injury that reduces the tumor mass. (b) Mobilization of T regulatory (Treg) cells and myeloid‑derived suppressor cells (MDSCs) to the tumor mass, as well as changes
in the phenotypes of tumor‑associated macrophages (TAM) result in pro‑tumorigenic regulation with increases in tumor cell proliferation, angiogenesis, and invasion
numbers than tumor associated macrophages, suggesting many effects, TGF-β1 in the tumor microenvironment
that they might play a key role in glioma invasion. [79] is an important regulator of glioma invasion. [87,88]
The overactive JNK signaling in NF-1 heterozygous
Recent genetic microarray analyses of glioma patient microglia may lead to a constitutively active state of
tumors have revealed variations between glioma microglia based on morphology and expression profiles.
subtype progression, invasion, and response to therapy. The existence of activated macrophage/microglia
In patients enrolled in a phase I dendritic cell (DC) within the GBM tumor mass may facilitate a relatively
vaccine therapy clinical trial, we identified significant more favorable immunogenic microenvironment that
trends in the mesenchymal glioma subtype, including maintains T cell activation once they are mobilized
its progression and its particular responsiveness to tumor by DC vaccination. This theory underscores
to treatment. [80] It may be worth exploring if the the crucial role that microglia may play in the tumor
mesenchymal subgroup of GBM patients have tumor microenvironment by potentiating the immune
cells carrying LOH in the neurofibromatosis-1 (NF-1) responses against tumor cells. Indeed, it has been
gene, also have NF-1 heterozygous microglia populating proposed that modified microglia may have benefit for
the GBM tumor microenvironment. NF-1 heterozygous glioma treatment. [89,90]
microglia are essential in driving optic nerve astroglioma
[81]
with NF-1 LOH. Further, NF-1 heterozygous microglia Indeed, modulating the microglia in the tumor
drive optic nerve glioma by facilitating a relatively microenvironment of wild type NF-1 patients may
more “type 1” chronic inflammatory microenvironment prove to be an important aspect to glioma therapy.
through increased c-Jun-NH -kinase (JNK) signaling IL-10-mediated inhibition of NF-κB heterodimer
2
leading to the constitutive expression of higher levels of (p50/p65) formation leads to an over-expression of
pro-inflammatory cytokines and proteins TNF-α, IL-1, the NF-κB homodimer (p50/p50), which prevents
iNOS, and Cox2. The JNK and ERK1/2 pathway is not transcription elongation of various genes encoding
[82]
only responsible for the expression of pro-inflammatory pro-inflammatory cytokines. This is predominantly
cytokines, but also for the repression of the responsible for the tolerant M2 macrophage phenotype
transcriptional potential of Smad3 activated by TGF-β encountered in the microenvironment of wild
as well. [83-85] Conversely, TGF-β1 mediates its effects type NF-1 patients. [91-93] Interestingly, IL-10 is a cytokine
through inhibition of the ERK pathway. [86] Among its translated in tandem with other pro-inflammatory
70 Neuroimmunol Neuroinflammation | Volume 1 | Issue 2 | September 2014