Page 144 - Read Online
P. 144
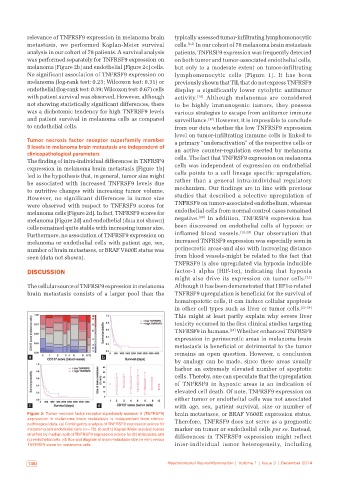
relevance of TNFRSF9 expression in melanoma brain typically assessed tumor‑infiltrating lymphomonocytic
metastasis, we performed Kaplan‑Meier survival cells. [2,3] In our cohort of 78 melanoma brain metastasis
analysis in our cohort of 78 patients. A survival analysis patients, TNFRSF9 expression was frequently detected
was performed separately for TNFRSF9 expression on on both tumor and tumor‑associated endothelial cells,
melanoma [Figure 2b] and endothelial [Figure 2c] cells. but only to a moderate extent on tumor‑infiltrating
No significant association of TNFRSF9 expression on lymphomonocytic cells [Figure 1]. It has been
melanoma (log‑rank test: 0.23; Wilcoxon test: 0.31) or previously shown that TIL that do not express TNFRSF9
endothelial (log‑rank test: 0.39; Wilcoxon test: 0.67) cells display a significantly lower cytolytic antitumor
with patient survival was observed. However, although activity. [16] Although melanomas are considered
not showing statistically significant differences, there to be highly immunogenic tumors, they possess
was a dichotomic tendency for high TNFRSF9 levels various strategies to escape from antitumor immune
and patient survival in melanoma cells as compared surveillance. [17] However, it is impossible to conclude
to endothelial cells. from our data whether the low TNFRSF9 expression
level on tumor‑infiltrating immune cells is linked to
Tumor necrosis factor receptor superfamily member a primary “underactivation” of the respective cells or
9 levels in melanoma brain metastasis are independent of an active counter‑regulation exerted by melanoma
clinicopathological parameters
The finding of intra‑individual differences in TNFRSF9 cells. The fact that TNFRSF9 expression on melanoma
expression in melanoma brain metastasis [Figure 1b] cells was independent of expression on endothelial
led to the hypothesis that, in general, tumor size might cells points to a cell lineage specific upregulation,
be associated with increased TNFRSF9 levels due rather than a general intra‑individual regulatory
to nutritive changes with increasing tumor volume. mechanism. Our findings are in line with previous
However, no significant differences in tumor size studies that described a selective upregulation of
were observed with respect to TNFRSF9 scores for TNFRSF9 on tumor‑associated endothelium, whereas
melanoma cells [Figure 2d]. In fact, TNFRSF9 scores for endothelial cells from normal control cases remained
melanoma [Figure 2d] and endothelial (data not shown) negative. [18] In addition, TNFRSF9 expression has
cells remained quite stable with increasing tumor size. been discovered on endothelial cells of hypoxic or
Furthermore, no association of TNFRSF9 expression on inflamed blood vessels. [19,20] Our observation that
melanoma or endothelial cells with patient age, sex, increased TNFRSF9 expression was especially seen in
number of brain metastases, or BRAF V600E status was perinecrotic areas‑and also with increasing distance
seen (data not shown). from blood vessels‑might be related to the fact that
TNFRSF9 is also upregulated via hypoxia inducible
DISCUSSION factor‑1 alpha (HIF‑1α), indicating that hypoxia
might also drive its expression on tumor cells. [21]
The cellular source of TNFRSF9 expression in melanoma Although it has been demonstrated that HIF1α‑related
brain metastasis consists of a larger pool than the TNFRSF9 upregulation is beneficial for the survival of
hematopoietic cells, it can induce cellular apoptosis
in other cell types such as liver or tumor cells. [22‑24]
This might at least partly explain why severe liver
toxicity occurred in the first clinical studies targeting
TNFRSF9 in humans. [25] Whether enhanced TNFRSF9
expression in perinecrotic areas in melanoma brain
metastasis is beneficial or detrimental to the tumor
remains an open question. However, a conclusion
a b
by analogy can be made, since these areas usually
harbor an extremely elevated number of apoptotic
cells. Thereby, one can speculate that the upregulation
of TNFRSF9 in hypoxic areas is an indication of
elevated cell death. Of note, TNFRSF9 expression on
either tumor or endothelial cells was not associated
c d with age, sex, patient survival, size or number of
Figure 2: Tumor necrosis factor receptor superfamily member 9 (TNFRSF9) brain metastases, or BRAF V600E expression status.
expression in melanoma brain metastasis is independent from clinico‑ Therefore, TNFRSF9 does not serve as a prognostic
pathological data. (a) Contingency analysis of TNFRSF9 expression scores for
melanoma and endothelial cells (n = 78). (b and c) Kaplan‑Meier survival curves marker on tumor or endothelial cells per se. Instead,
stratified by median split of TNFRSF9 expression scores for (b) melanoma and differences in TNFRSF9 expression might reflect
(c) endothelial cells. (d) Box‑plot diagram of brain metastasis size (in mm) versus
TNFRSF9 score for melanoma cells inter‑individual tumor heterogeneity, including
138 Neuroimmunol Neuroinflammation | Volume 1 | Issue 3 | December 2014