Page 42 - Read Online
P. 42
Kalloo et al. Metab Target Organ Damage 2023;3:7 https://dx.doi.org/10.20517/mtod.2022.26 Page 3 of 19
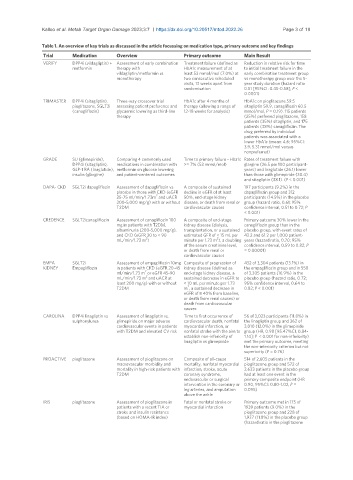
Table 1. An overview of key trials as discussed in the article focussing on medication type, primary outcome and key findings
Trial Medication Overview Primary outcome Main Result
VERIFY DPP4i (vildagliptin) + Assessment of early combination Treatment failure (defined as Reduction in relative risk for time
metformin therapy with HbA1c measurement of at to initial treatment failure in the
vildagliptin/metformin vs. least 53 mmol/mol (7.0%) at early combination treatment group
monotherapy two consecutive scheduled vs monotherapy group over the 5-
visits, 13 weeks apart from year study duration (hazard ratio
randomisation 0.51 [95%CI: 0.45-0.58]; P <
0.0001)
TRIMASTER DPP4i (sitagliptin), Three-way crossover trial HbA1c after 4 months of HbA1c on pioglitazone 59.5
pioglitazone, SGLT2i assessing patient preference and therapy (allowing a range of sitagliptin 59.9, canagliflozin 60.5
(canagliflozin) glycaemic lowering as third-line 12-18 weeks for analysis) mmol/mol, P = 0.19). 115 patients
therapy (25%) preferred pioglitazone, 158
patients (35%) sitagliptin, and 175
patients (38%) canagliflozin. The
drug preferred by individual
patients was associated with a
lower HbA1c (mean: 4.6; 95%CI:
3.9, 5.3) mmol/mol versus
nonpreferred)
GRADE SU (glimepiride), Comparing 4 commonly used Time to primary failure - Hba1c Rates of treatment failure with
DPP4i (sitagliptin), medications in combination with >= 7% (53 mmol/mol) glargine (26.5 per 100 participant-
GLP-1 RA (liraglutide), metformin on glucose lowering years) and liraglutide (26.1) lower
insulin (glargine) and patient-centered outcomes than those with glimepiride (30.4)
and sitagliptin (38.1). (P < 0.001)
DAPA- CKD SGLT2i dapagliflozin Assessment of dapagliflozin vs A composite of sustained 197 participants (9.2%) in the
placebo in those with CKD (eGFR decline in eGFR of at least dapagliflozin group and 312
2
25-75 ml/min/1.73m and uACR 50%, end-stage kidney participants (14.5%) in the placebo
200-5,000 mg/g) with or without disease, or death from renal or group (hazard ratio, 0.61; 95%
T2DM cardiovascular causes confidence interval, 0.51 to 0.72; P
< 0.001)
CREDENCE SGLT2icanagliflozin Assessment of canagliflozin 100 A composite of end-stage Primary outcome 30% lower in the
mg in patients with T2DM, kidney disease (dialysis, canagliflozin group than in the
albuminuria (200-5,000 mg/g), transplantation, or a sustained placebo group, with event rates of
and CKD (eGFR 30 to < 90 estimated GFR of < 15 mL per 43.2 and 61.2 per 1,000 patient-
2
2
mL/min/1.73 m ) minute per 1.73 m ), a doubling years (hazard ratio, 0.70; 95%
of the serum creatinine level, confidence interval, 0.59 to 0.82; P
or death from renal or = 0.00001)
cardiovascular causes
EMPA SGLT2i Assessment of empagliflozin 10mg Composite of progression of 432 of 3,304 patients (13.1%) in
KIDNEY Empagliflozin in patients with CKD (eGFR 20-45 kidney disease (defined as the empagliflozin group and in 558
2
ml/min/1.73 m or eGFR 45-90 end-stage kidney disease, a of 3,305 patients (16.9%) in the
2
mL/min/1.73 m and uACR at sustained decrease in eGFR to placebo group (hazard ratio, 0.72;
least 200 mg/g) with or without < 10 mL per minute per 1.73 95% confidence interval, 0.64 to
2
T2DM m , a sustained decrease in 0.82; P < 0.001)
eGFR of ≥ 40% from baseline,
or death from renal causes) or
death from cardiovascular
causes
CAROLINA DPP4i linagliptin vs Assessment of linagliptin vs. Time to first occurrence of 56 of 3,023 participants (11.8%) in
sulphonylurea glimepiride on major adverse cardiovascular death, nonfatal the linagliptin group and 362 of
cardiovascular events in patients myocardial infarction, or 3,010 (12.0%) in the glimepiride
with T2DM and elevated CV risk nonfatal stroke with the aim to group (HR, 0.98 [95.47%CI, 0.84-
establish non-inferiority of 1.14]; P < 0.001 for non-inferiority)
linagliptin vs glimepiride met the primary outcome, meeting
the non-inferiority criterion but not
superiority (P = 0.76)
PROACTIVE pioglitazone Assessment of pioglitazone on Composite of all-cause 514 of 2,605 patients in the
macrovascular morbidity and mortality, nonfatal myocardial pioglitazone group and 572 of
mortality in high-risk patients with infarction, stroke, acute 2,633 patients in the placebo group
T2DM coronary syndrome, had at least one event in the
endovascular or surgical primary composite endpoint (HR
intervention in the coronary or 0.90, 95%CI: 0.80-1.02, P =
leg arteries, and amputation 0.095)
above the ankle
IRIS pioglitazone Assessment of pioglitazone in Fatal or nonfatal stroke or Primary outcome met in 175 of
patients with a recent TIA or myocardial infarction 1939 patients (9.0%) in the
stroke and insulin resistance pioglitazone group and 228 of
(based on HOMA-IR index) 1,937 (11.8%) in the placebo group
(hazard ratio in the pioglitazone