Page 43 - Read Online
P. 43
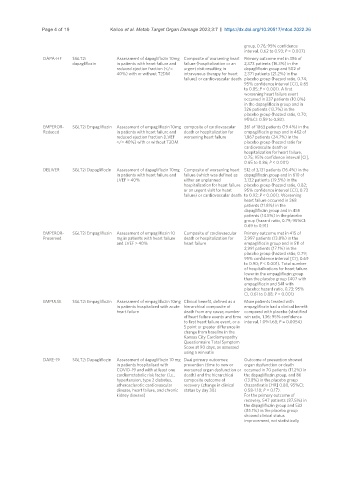
Page 4 of 19 Kalloo et al. Metab Target Organ Damage 2023;3:7 https://dx.doi.org/10.20517/mtod.2022.26
group, 0.76; 95% confidence
interval, 0.62 to 0.93; P = 0.007)
DAPA-HF SGLT2i Assessment of dapagliflozin 10mg Composite of worsening heart Primary outcome met in 386 of
dapagliflozin in patients with heart failure and failure (hospitalization or an 2,373 patients (16.3%) in the
reduced ejection fraction (</= urgent visit resulting in dapagliflozin group and 502 of
40%) with or without T2DM intravenous therapy for heart 2,371 patients (21.2%) in the
failure) or cardiovascular death placebo group (hazard ratio, 0.74;
95% confidence interval [CI], 0.65
to 0.85; P < 0.001). A first
worsening heart failure event
occurred in 237 patients (10.0%)
in the dapagliflozin group and in
326 patients (13.7%) in the
placebo group (hazard ratio, 0.70;
95%CI: 0.59 to 0.83).
EMPEROR- SGLT2i Empagliflozin Assessment of empagliflozin 10mg composite of cardiovascular 361 of 1863 patients (19.4%) in the
Reduced in patients with heart failure and death or hospitalization for empagliflozin group and in 462 of
reduced ejection fraction (LVEF worsening heart failure 1,867 patients (24.7%) in the
</= 40%) with or without T2DM placebo group (hazard ratio for
cardiovascular death or
hospitalization for heart failure,
0.75; 95% confidence interval [CI],
0.65 to 0.86; P < 0.001)
DELIVER SGLT2i Dapagliflozin Assessment of dapagliflozin 10mg Composite of worsening heart 512 of 3,131 patients (16.4%) in the
in patients with heart failure and failure (which was defined as dapagliflozin group and in 610 of
LVEF > 40% either an unplanned 3,132 patients (19.5%) in the
hospitalization for heart failure placebo group (hazard ratio, 0.82;
or an urgent visit for heart 95% confidence interval [CI], 0.73
failure) or cardiovascular death to 0.92; P < 0.001). Worsening
heart failure occurred in 368
patients (11.8%) in the
dapagliflozin group and in 455
patients (14.5%) in the placebo
group (hazard ratio, 0.79; 95%CI:
0.69 to 0.91)
EMPEROR- SGLT2i Empagliflozin Assessment of empagliflozin 10 Composite of cardiovascular Primary outcome met in 415 of
Preserved mg in patients with heart failure death or hospitalization for 2,997 patients (13.8%) in the
and LVEF > 40% heart failure empagliflozin group and in 511 of
2,991 patients (17.1%) in the
placebo group (hazard ratio, 0.79;
95% confidence interval [CI], 0.69
to 0.90; P < 0.001). Total number
of hospitalizations for heart failure
lower in the empagliflozin group
than the placebo group (407 with
empagliflozin and 541 with
placebo; hazard ratio, 0.73; 95%
CI, 0.61 to 0.88; P < 0.001)
EMPULSE SGLT2i Empagliflozin Assessment of empagliflozin 10mg Clinical benefit, defined as a More patients treated with
in patients hospitalized with acute hierarchical composite of empagliflozin had a clinical benefit
heart failure death from any cause, number compared with placebo (stratified
of heart failure events and time win ratio, 1.36; 95% confidence
to first heart failure event, or a interval, 1.09-1.68; P = 0.0054)
5 point or greater difference in
change from baseline in the
Kansas City Cardiomyopathy
Questionnaire Total Symptom
Score at 90 days, as assessed
using a win ratio
DARE-19 SGLT2i Dapagliflozin Assessment of dapagliflozin 10 mg Dual primary outcomes: Outcome of prevention showed
in patients hospitalised with prevention (time to new or organ dysfunction or death
COVID-19 and with at least one worsened organ dysfunction or occurred in 70 patients (11.2%) in
cardiometabolic risk factor (i.e., death) and the hierarchical the dapagliflozin group, and 86
hypertension, type 2 diabetes, composite outcome of (13.8%) in the placebo group
atherosclerotic cardiovascular recovery (change in clinical (hazard ratio [HR] 0.80, 95%CI:
disease, heart failure, and chronic status by day 30) 0.58-1.10; P = 0.17).
kidney disease) For the primary outcome of
recovery, 547 patients (87.5%) in
the dapagliflozin group and 532
(85.1%) in the placebo group
showed clinical status
improvement, not statistically