Page 602 - Read Online
P. 602
Saxena et al. Mini-invasive Surg 2020;4:62 I http://dx.doi.org/10.20517/2574-1225.2020.68 Page 11 of 15
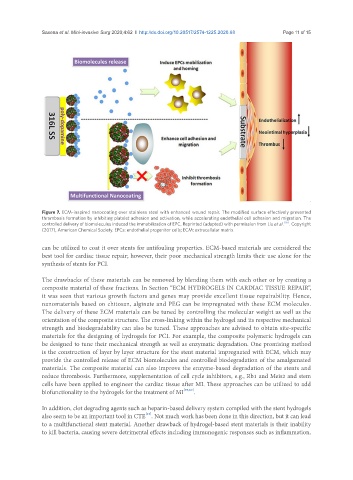
Figure 7. ECM-inspired nanocoating over stainless steel with enhanced wound repair. The modified surface effectively prevented
thrombosis formation by inhibiting platelet adhesion and activation, while accelerating endothelial cell adhesion and migration. The
controlled delivery of biomolecules induced the immobilization of EPC. Reprinted (adapted) with permission from Liu et al. [55] . Copyright
(2017), American Chemical Society. EPCs: endothelial progenitor cells; ECM: extracellular matrix
can be utilized to coat it over stents for antifouling properties. ECM-based materials are considered the
best tool for cardiac tissue repair; however, their poor mechanical strength limits their use alone for the
synthesis of stents for PCI.
The drawbacks of these materials can be removed by blending them with each other or by creating a
composite material of these fractions. In Section “ECM HYDROGELS IN CARDIAC TISSUE REPAIR”,
it was seen that various growth factors and genes may provide excellent tissue repairability. Hence,
nanomaterials based on chitosan, alginate and PEG can be impregnated with these ECM molecules.
The delivery of these ECM materials can be tuned by controlling the molecular weight as well as the
orientation of the composite structure. The cross-linking within the hydrogel and its respective mechanical
strength and biodegradability can also be tuned. These approaches are advised to obtain site-specific
materials for the designing of hydrogels for PCI. For example, the composite polymeric hydrogels can
be designed to tune their mechanical strength as well as enzymatic degradation. One promising method
is the construction of layer by layer structure for the stent material impregnated with ECM, which may
provide the controlled release of ECM biomolecules and controlled biodegradation of the amalgamated
materials. The composite material can also improve the enzyme-based degradation of the stents and
reduce thrombosis. Furthermore, supplementation of cell cycle inhibitors, e.g., Rb1 and Meis2 and stem
cells have been applied to engineer the cardiac tissue after MI. These approaches can be utilized to add
biofunctionality to the hydrogels for the treatment of MI [59,60] .
In addition, clot degrading agents such as heparin-based delivery system compiled with the stent hydrogels
[61]
also seem to be an important tool in CTE . Not much work has been done in this direction, but it can lead
to a multifunctional stent material. Another drawback of hydrogel-based stent materials is their inability
to kill bacteria, causing severe detrimental effects including immunogenic responses such as inflammation,