Page 27 - Read Online
P. 27
Kumar et al. Mini-invasive Surg 2018;2:19 I http://dx.doi.org/10.20517/2574-1225.2018.26 Page 5 of 15
Table 1. Case series of laparoscopic pelvic exenteration and outcomes
Study Year Number of Median Median Conversion Overall Hospital R0 status Follow-up
patients (n) operative time, blood loss, to open (%) complications stay (%) (months)
min (range) mL (range) (%) (days)
Uehara 2015 6/48 935 830 16.7 66.7 27 77.8 NR
et al. [29] (LPE/OPE) (716-1219) (283-5225) (23-53)
Ogura 2016 13/18 829 930 0 61.5 29 100 NR
et al. [32] (LPE/OPE)
Pokharkar 2018 10 (LPE) 547 1000 0 20 14.6 100 NR
et al. [38] (300-2000) (9-25)
LPE: laparoscopic pelvic exenteration; OPE: open pelvic exenteration; NR: not reported
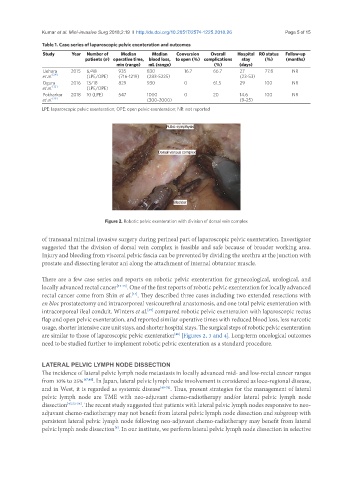
Pubic symphysis
Dorsal venous complex
Bladder
Figure 2. Robotic pelvic exenteration with division of dorsal vein complex
of transanal minimal invasive surgery during perineal part of laparoscopic pelvic exenteration. Investigator
suggested that the division of dorsal vein complex is feasible and safe because of broader working area.
Injury and bleeding from visceral pelvic fascia can be prevented by dividing the urethra at the junction with
prostate and dissecting levator ani along the attachment of internal obturator muscle.
There are a few case series and reports on robotic pelvic exenteration for gynecological, urological, and
locally advanced rectal cancer [41-43] . One of the first reports of robotic pelvic exenteration for locally advanced
rectal cancer come from Shin et al. . They described three cases including two extended resections with
[44]
en bloc prostatectomy and intracorporeal vesicourethral anastomosis, and one total pelvic exenteration with
intracorporeal ileal conduit. Winters et al. compared robotic pelvic exenteration with laparoscopic rectus
[45]
flap and open pelvic exenteration, and reported similar operative times with reduced blood loss, less narcotic
usage, shorter intensive care unit stays, and shorter hospital stays. The surgical steps of robotic pelvic exenteration
are similar to those of laparoscopic pelvic exenteration [Figures 2, 3 and 4]. Long-term oncological outcomes
[46]
need to be studied further to implement robotic pelvic exenteration as a standard procedure.
LATERAL PELVIC LYMPH NODE DISSECTION
The incidence of lateral pelvic lymph node metastasis in locally advanced mid- and low-rectal cancer ranges
from 10% to 25% [47,48] . In Japan, lateral pelvic lymph node involvement is considered as loco-regional disease,
and in West, it is regarded as systemic disease [49-51] . Thus, present strategies for the management of lateral
pelvic lymph node are TME with neo-adjuvant chemo-radiotherapy and/or lateral pelvic lymph node
dissection [47,52-54] . The recent study suggested that patients with lateral pelvic lymph nodes responsive to neo-
adjuvant chemo-radiotherapy may not benefit from lateral pelvic lymph node dissection and subgroup with
persistent lateral pelvic lymph node following neo-adjuvant chemo-radiotherapy may benefit from lateral
pelvic lymph node dissection . In our institute, we perform lateral pelvic lymph node dissection in selective
[8]