Page 10 - Read Online
P. 10
Page 4 of 10 Quenelle et al. J Unexplored Med Data 2018;3:7 I http://dx.doi.org/10.20517/2572-8180.2018.02
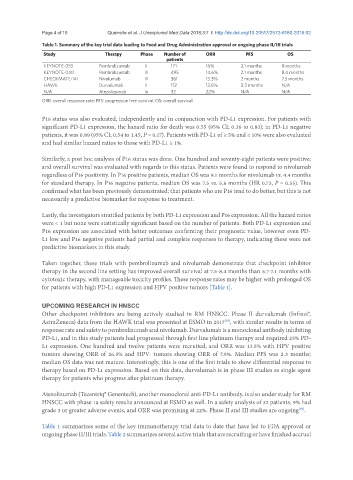
Table 1. Summary of the key trial data leading to Food and Drug Administration approval or ongoing phase II/III trials
Study Therapy Phase Number of ORR PFS OS
patients
KEYNOTE-055 Pembrolizumab II 171 16% 2.1 months 8 months
KEYNOTE-040 Pembrolizumab III 495 14.6% 2.1 months 8.4 months
CHECKMATE-141 Nivolumab III 361 13.3% 2 months 7.5 months
HAWK Durvalumab II 112 13.5% 2.3 months N/A
N/A Atezolizumab Ia 32 22% N/A N/A
ORR: overall response rate; PFS: progression free survival; OS: overall survival
P16 status was also evaluated, independently and in conjunction with PD-L1 expression. For patients with
significant PD-L1 expression, the hazard ratio for death was 0.55 (95% CI; 0.36 to 0.83); in PD-L1 negative
patients, it was 0.89 (95% CI; 0.54 to 1.45, P = 0.17). Patients with PD-L1 of ≥ 5% and ≥ 10% were also evaluated
and had similar hazard ratios to those with PD-L1 ≥ 1%.
Similarly, a post hoc analysis of P16 status was done. One hundred and seventy-eight patients were positive;
and overall survival was evaluated with regards to this status. Patients were found to respond to nivolumab
regardless of P16 positivity. In P16 positive patients, median OS was 9.1 months for nivolumab vs. 4.4 months
for standard therapy. In P16 negative patients, median OS was 7.5 vs. 5.8 months (HR 0.73, P = 0.55). This
confirmed what has been previously demonstrated; that patients who are P16 tend to do better, but this is not
necessarily a predictive biomarker for response to treatment.
Lastly, the investigators stratified patients by both PD-L1 expression and P16 expression. All the hazard ratios
were < 1 but none were statistically significant based on the number of patients. Both PD-L1 expression and
P16 expression are associated with better outcomes confirming their prognostic value, however even PD-
L1 low and P16 negative patients had partial and complete responses to therapy, indicating these were not
predictive biomarkers in this study.
Taken together, these trials with pembrolizumab and nivolumab demonstrate that checkpoint inhibitor
therapy in the second line setting has improved overall survival at 7.5-8.4 months than 5.7-7.1 months with
cytotoxic therapy, with manageable toxicity profiles. These response rates may be higher with prolonged OS
for patients with high PD-L1 expression and HPV positive tumors [Table 1].
UPCOMING RESEARCH IN HNSCC
Other checkpoint inhibitors are being actively studied in RM HNSCC. Phase II durvalumab (Infinzi®,
AstraZeneca) data from the HAWK trial was presented at ESMO in 2017 , with similar results in terms of
[18]
response rate and safety to pembrolizumab and nivolumab. Durvalumab is a monoclonal antibody inhibiting
PD-L1, and in this study patients had progressed through first line platinum therapy and required 25% PD-
L1 expression. One hundred and twelve patients were recruited, and ORR was 13.5% with HPV positive
tumors showing ORR of 26.5% and HPV- tumors showing ORR of 7.9%. Median PFS was 2.3 months;
median OS data was not mature. Interestingly, this is one of the first trials to show differential response to
therapy based on PD-L1 expression. Based on this data, durvalumab is in phase III studies as single agent
therapy for patients who progress after platinum therapy.
Atezolizumab (Tecentriq® Genentech), another monoclonal anti-PD-L1 antibody, is also under study for RM
HNSCC with phase 1a safety results announced at ESMO as well. In a safety analysis of 32 patients, 9% had
grade 3 or greater adverse events, and ORR was promising at 22%. Phase II and III studies are ongoing .
[19]
Table 1 summarizes some of the key immunotherapy trial data to date that have led to FDA approval or
ongoing phase II/III trials. Table 2 summarizes several active trials that are recruiting or have finished accrual