Page 12 - Read Online
P. 12
Page 6 of 10 Quenelle et al. J Unexplored Med Data 2018;3:7 I http://dx.doi.org/10.20517/2572-8180.2018.02
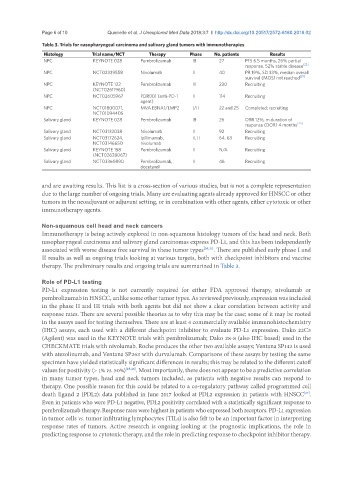
Table 3. Trials for nasopharyngeal carcinoma and salivary gland tumors with immunotherapies
Histology Trial name/NCT Therapy Phase No. patients Results
NPC KEYNOTE 028 Pembrolizumab IB 27 PFS 6.5 months, 26% partial
response, 52% stable disease [22]
NPC NCT02339558 Nivolumab II 40 PR 19%, SD 33%, median overall
survival (MOS) not reached [23]
NPC KEYNOTE 122 Pembrolizumab III 230 Recruiting
(NCT02611960)
NPC NCT02605967 PDR001 (anti-PD-1 II 114 Recruiting
agent)
NPC NCT01800071, MVA EBNA1/LMP2 I/II 22 and 25 Completed; recruiting
NCT01094405
Salivary gland KEYNOTE 028 Pembrolizumab IB 26 ORR 12%, m duration of
response (DOR) 4 months [24]
Salivary gland NCT03132038 Nivolumab II 92 Recruiting
Salivary gland NCT03172624, Ipilimumab, II, II 64, 63 Recruiting
NCT03146650 nivolumab
Salivary gland KEYNOTE 158 Pembrolizumab II N/A Recruiting
(NCT02628067)
Salivary gland NCT03360890 Pembrolizumab, II 46 Recruiting
docetaxel
and are awaiting results. This list is a cross-section of various studies, but is not a complete representation
due to the large number of ongoing trials. Many are evaluating agents already approved for HNSCC or other
tumors in the neoadjuvant or adjuvant setting, or in combination with other agents, either cytotoxic or other
immunotherapy agents.
Non-squamous cell head and neck cancers
Immunotherapy is being actively explored in non-squamous histology tumors of the head and neck. Both
nasopharyngeal carcinoma and salivary gland carcinomas express PD-L1, and this has been independently
associated with worse disease free survival in these tumor types [20,21] . There are published early phase I and
II results as well as ongoing trials looking at various targets, both with checkpoint inhibitors and vaccine
therapy. The preliminary results and ongoing trials are summarized in Table 3.
Role of PD-L1 testing
PD-L1 expression testing is not currently required for either FDA approved therapy, nivolumab or
pembrolizumab in HNSCC, unlike some other tumor types. As reviewed previously, expression was included
in the phase II and III trials with both agents but did not show a clear correlation between activity and
response rates. There are several possible theories as to why this may be the case; some of it may be rooted
in the assays used for testing themselves. There are at least 4 commercially available immunohistochemistry
(IHC) assays, each used with a different checkpoint inhibitor to evaluate PD-L1 expression. Dako 22C3
(Agilent) was used in the KEYNOTE trials with pembrolizumab; Dako 28-8 (also IHC based) used in the
CHECKMATE trials with nivolumab. Roche produces the other two available assays; Ventana SP142 is used
with atezolizumab, and Ventana SP263 with durvalumab. Comparisons of these assays by testing the same
specimen have yielded statistically significant differences in results; this may be related to the different cutoff
values for positivity (> 1% vs. 50%) [25,26] . Most importantly, there does not appear to be a predictive correlation
in many tumor types, head and neck tumors included, as patients with negative results can respond to
therapy. One possible reason for this could be related to a co-regulatory pathway called programmed cell
death ligand 2 (PDL2): data published in June 2017 looked at PDL2 expression in patients with HNSCC .
[27]
Even in patients who were PD-L1 negative, PDL2 positivity correlated with a statistically significant response to
pembrolizumab therapy. Response rates were highest in patients who expressed both receptors. PD-L1 expression
in tumor cells vs. tumor infiltrating lymphocytes (TILs) is also felt to be an important factor in interpreting
response rates of tumors. Active research is ongoing looking at the prognostic implications, the role in
predicting response to cytotoxic therapy, and the role in predicting response to checkpoint inhibitor therapy.