Page 11 - Read Online
P. 11
Quenelle et al. J Unexplored Med Data 2018;3:7 I http://dx.doi.org/10.20517/2572-8180.2018.02 Page 5 of 10
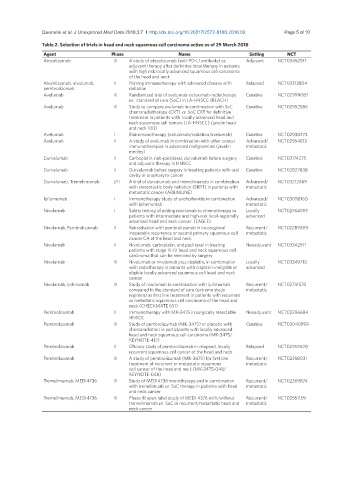
Table 2. Selection of trials in head and neck squamous cell carcinoma active as of 29 March 2018
Agent Phase Name Setting NCT
Atezolizumab III A study of atezolizumab (anti-PD-L1 antibody) as Adjuvant NCT03452137
adjuvant therapy after definitive local therapy in patients
with high risk locally advanced squamous cell carcinoma
of the head and neck
Atezolizumab, nivolumab, II Priming immunotherapy with advanced disease with Relapsed NCT03313804
pembrolizomab radiation
Avelumab III Randomized trial of avelumab-cetuximab-radiotherapy Curative NCT02999087
vs. standard of care (SoC) in LA-HNSCC (REACH)
Avelumab III Study to compare avelumab in combination with SoC Curative NCT02952586
chemoradiotherapy (CRT) vs. SoC CRT for definitive
treatment in patients with locally advanced head and
neck squamous cell tumors (LA-HNSCC) (javelin head
and neck 100)
Avelumab I Bioimmunotherapy (cetuximab/radiation/avelumab) Curative NCT02938273
Avelumab II A study of avelumab in combination with other cancer Advanced/ NCT02554812
immunotherapies in advanced malignancies (javelin metastatic
medley)
Durvalumab II Carboplatin, nab-paclitaxel, durvalumab before surgery Curative NCT03174275
and adjuvant therapy in HNSCC
Durvalumab II Durvalumab before surgery in treating patients with oral Curative NCT02827838
cavity or oropharynx cancer
Durvalumab, Tremelimumab I/II A trial of durvalumab and tremelimumab in combination Advanced/ NCT03212469
with stereotactic body radiation (SBRT) in patients with metastatic
metastatic cancer (ABBIMUNE)
Ipilumumab I Immunotherapy study of evofosfamide in combination Advanced/ NCT03098160
with ipilumumab metastatic
Nivolumab I Safety testing of adding nivolumab to chemotherapy in Locally NCT02764593
patients with intermediate and high-risk local-regionally advanced
advanced head and neck cancer (EAGLE)
Nivolumab, Pembrolizumab II Reirradiation with pembrolizumab in locoregional Recurrent/ NCT02289209
inoperable recurrence or second primary squamous cell metastatic
cancer CA of the head and neck
Nivolumab II Nivolumab, carboplatin, and paclitaxel in treating Neoadjuvant NCT03342911
patients with stage III-IV head and neck squamous cell
carcinoma that can be removed by surgery
Nivolumab III Nivolumab or nivolumab plus cisplatin, in combination Locally NCT03349710
with radiotherapy in patients with cisplatin-ineligible or advanced
eligible locally advanced squamous cell head and neck
cancer
Nivolumab, Ipilimumab III Study of nivolumab in combination with ipilimumab Recurrent/ NCT02741570
compared to the standard of care (extreme study metastatic
regimen) as first line treatment in patients with recurrent
or metastatic squamous cell carcinoma of the head and
neck (CHECKMATE 651)
Pembrolizumab II Immunotherapy with MK-3475 in surgically resectable Neoadjuvant NCT02296684
HNSCC
Pembrolizumab III Study of pembrolizumab (MK-3475) or placebo with Curative NCT03040999
chemoradiation in participants with locally advanced
head and neck squamous cell carcinoma (MK-3475/
KEYNOTE-412)
Pembrolizumab II Efficacy study of pembrolizumab in relapsed, locally Relapsed NCT02769520
recurrent squamous cell cancer of the head and neck
Pembrolizumab III A study of pembrolizumab (MK-3475) for first line Recurrent/ NCT02358031
treatment of recurrent or metastatic squamous metastatic
cell cancer of the head and neck (MK-3475-048/
KEYNOTE-048)
Tremelimumab, MEDI4736 III Study of MEDI4736 monotherapy and in combination Recurrent/ NCT02369874
with tremelimuab vs. SoC therapy in patients with head metastatic
and neck cancer
Tremelimumab, MEDI4736 III Phase III open label study of MEDI 4376 with/without Recurrent/ NCT02551159
tremelimumab vs. SoC in recurrent/metastatic head and metastatic
neck cancer