Page 29 - Read Online
P. 29
Shaughnessy et al. J Transl Genet Genom 2018;2:14. I https://doi.org/10.20517/jtgg.2018.25 Page 3 of 13
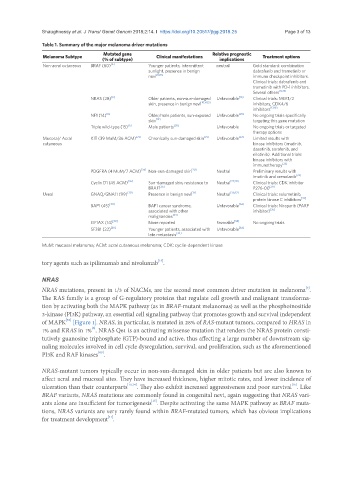
Table 1. Summary of the major melanoma driver mutations
Melanoma Subtype Mutated gene Clinical manifestations Relative prognostic Treatment options
(% of subtype) implications
Non-acral cutaneous BRAF (60) [6] Younger patients, intermittent neutral Gold standard: combination
sunlight, presence in benign dabrafenib and trametinib or
nevi [10,11] immune checkpoint inhibitors.
Clinical trials: dabrafenib and
trametinib with PD-1 inhibitors.
Several others [12,13]
NRAS (28) [6] Older patients, non-sun-damaged Unfavorable [16] Clinical trials: MEK1/2
skin, presence in benign nevi [10,14,15] inhibitors, CDK4/6
inhibitors [17,18]
NF1 (14) [6] Older/male patients, sun-exposed Unfavorable [20] No ongoing trials specifically
skin [19] targeting this gene mutation
Triple wild-type (15) [6] Male patients [20] Unfavorable No ongoing trials or targeted
therapy options
Mucosal/ Acral KIT (39 MuM/36 ACM) [21] Chronically sun-damaged skin [21] Unfavorable [22] Limited results with
cutaneous kinase inhibitors (imatinib,
dasatinib, sorafenib, and
nilotinib). Additional trials:
kinase inhibitors with
immunotherapy [23]
PDGFRA (4 MuM/7 ACM) [24] Non-sun-damaged skin [24] Neutral Preliminary results with
imatinib and crenolanib [25]
Cyclin D1 (45 ACM) [26] Sun-damaged skin, resistance to Neutral [27,28] Clinical trials: CDK inhibitor
BRAFi [26] P276-00 [29]
Uveal GNAQ/GNA11 (99) [30] Presence in benign nevi [31] Neutral [30,32] Clinical trials: solumetinib,
protein kinase C inhibitors [33]
BAP1 (45) [30] BAP1 cancer syndrome, Unfavorable [34] Clinical trials: Niraparib (PARP
associated with other inhibitor) [35]
malignancies [34]
EIF1AX (14) [30] None reported Favorable [34] No ongoing trials
SF3B1 (22) [30] Younger patients, associated with Unfavorable [34]
late metastasis [34]
MuM: mucosal melanoma; ACM: acral cutaneous melanoma; CDK: cyclin-dependent kinase
[13]
tory agents such as ipilimumab and nivolumab .
NRAS
[6]
NRAS mutations, present in 1/3 of NACMs, are the second most common driver mutation in melanoma .
The RAS family is a group of G-regulatory proteins that regulate cell growth and malignant transforma-
tion by activating both the MAPK pathway (as in BRAF-mutant melanomas) as well as the phosphoinositide
3-kinase (PI3K) pathway, an essential cell signaling pathway that promotes growth and survival independent
[39]
of MAPK [Figure 1]. NRAS, in particular, is mutated in 28% of RAS-mutant tumors, compared to HRAS in
[6]
1% and KRAS in 1% . NRAS Q61 is an activating missense mutation that renders the NRAS protein consti-
tutively guanosine triphosphate (GTP)-bound and active, thus affecting a large number of downstream sig-
naling molecules involved in cell cycle dysregulation, survival, and proliferation, such as the aforementioned
[40]
PI3K and RAF kinases .
NRAS-mutant tumors typically occur in non-sun-damaged skin in older patients but are also known to
affect acral and mucosal sites. They have increased thickness, higher mitotic rates, and lower incidence of
[16]
ulceration than their counterparts [10,14] . They also exhibit increased aggressiveness and poor survival . Like
BRAF variants, NRAS mutations are commonly found in congenital nevi, again suggesting that NRAS vari-
[15]
ants alone are insufficient for tumorigenesis . Despite activating the same MAPK pathway as BRAF muta-
tions, NRAS variants are very rarely found within BRAF-mutated tumors, which has obvious implications
[41]
for treatment development .