Page 31 - Read Online
P. 31
Shaughnessy et al. J Transl Genet Genom 2018;2:14. I https://doi.org/10.20517/jtgg.2018.25 Page 5 of 13
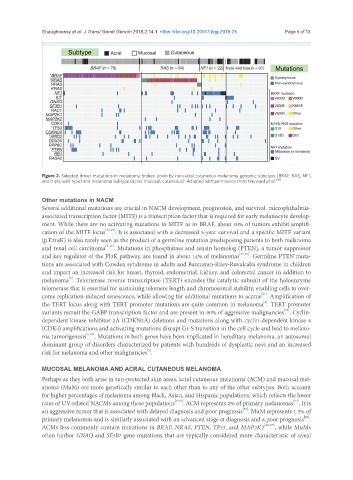
Figure 2. Selected driver mutations in melanoma broken down by non-acral cutaneous melanoma genomic subclass (BRAF, RAS, NF1,
and triple-wild type) and melanoma subtype (acral, mucosal, cutaneous). Adapted with permission from Hayward et al. [46]
Other mutations in NACM
Several additional mutations are crucial in NACM development, progression, and survival. microphthalmia-
associated transcription factor (MITF) is a transcription factor that is required for early melanocyte develop-
ment. While there are no activating mutations in MITF as in BRAF, about 10% of tumors exhibit amplifi-
cation of the MITF locus [47,48] . It is associated with a decreased 5-year survival and a specific MITF variant
(p.E318K) is also rarely seen as the product of a germline mutation predisposing patients to both melanoma
and renal cell carcinoma [5,32] . Mutations in phosphatase and tensin homolog (PTEN), a tumor suppressor
and key regulator of the PI3K pathway, are found in about 14% of melanomas [49,50] . Germline PTEN muta-
tions are associated with Cowden syndrome in adults and Bannayan-Riley-Ruvalcaba syndrome in children
and impart an increased risk for breast, thyroid, endometrial, kidney, and colorectal cancer in addition to
[5]
melanoma . Telomerase reverse transcriptase (TERT) encodes the catalytic subunit of the holoenzyme
telomerase that is essential for sustaining telomere length and chromosomal stability, enabling cells to over-
[51]
come replication-induced senescence, while allowing for additional mutations to accrue . Amplification of
[6]
the TERT locus along with TERT promoter mutations are quite common in melanoma . TERT promoter
[52]
variants recruit the GABP transcription factor and are present in 90% of aggressive malignancies . Cyclin-
dependent kinase inhibitor 2A (CDKN2A) deletions and mutations along with cyclin-dependent kinase 4
(CDK4) amplifications and activating mutations disrupt G1-S transition in the cell cycle and lead to melano-
ma tumorigenesis [6,46] . Mutations in both genes have been implicated in hereditary melanoma, an autosomal
dominant group of disorders characterized by patients with hundreds of dysplastic nevi and an increased
[5]
risk for melanoma and other malignancies .
MUCOSAL MELANOMA AND ACRAL CUTANEOUS MELANOMA
Perhaps as they both arise in sun-protected skin areas, acral cutaneous melanoma (ACM) and mucosal mel-
anoma (MuM) are more genetically similar to each other than to any of the other subtypes. Both account
for higher percentages of melanoma among Black, Asian, and Hispanic populations, which reflects the lower
[54]
rates of UV-related NACMs among these populations [6,53] . ACM represents 2% of primary melanomas . It is
[55]
an aggressive tumor that is associated with delayed diagnosis and poor prognosis . MuM represents 1.3% of
[56]
primary melanomas and is similarly associated with an advanced stage at diagnosis and a poor prognosis .
ACMs less commonly contain mutations in BRAF, NRAS, PTEN, TP53, and MAP2K2 [46,57] , while MuMs
often harbor GNAQ and SF3B1 gene mutations that are typically considered more characteristic of uveal