Page 30 - Read Online
P. 30
Page 4 of 13 Shaughnessy et al. J Transl Genet Genom 2018;2:14. I https://doi.org/10.20517/jtgg.2018.25
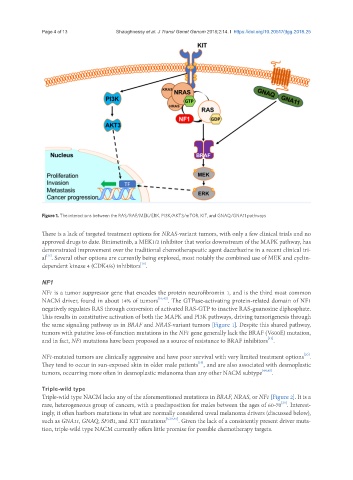
Figure 1. The interactions between the RAS/RAF/MEK/ERK, PI3K/AKT3/mTOR, KIT, and GNAQ/GNA11 pathways
There is a lack of targeted treatment options for NRAS-variant tumors, with only a few clinical trials and no
approved drugs to date. Binimetinib, a MEK1/2 inhibitor that works downstream of the MAPK pathway, has
demonstrated improvement over the traditional chemotherapeutic agent dacarbazine in a recent clinical tri-
[17]
al . Several other options are currently being explored, most notably the combined use of MEK and cyclin-
[18]
dependent kinase 4 (CDK4/6) inhibitors .
NF1
NF1 is a tumor suppressor gene that encodes the protein neurofibromin 1, and is the third most common
NACM driver, found in about 14% of tumors [16,42] . The GTPase-activating protein-related domain of NF1
negatively regulates RAS through conversion of activated RAS-GTP to inactive RAS-guanosine diphosphate.
This results in constitutive activation of both the MAPK and PI3K pathways, driving tumorigenesis through
the same signaling pathway as in BRAF and NRAS-variant tumors [Figure 1]. Despite this shared pathway,
tumors with putative loss-of-function mutations in the NF1 gene generally lack the BRAF (V600E) mutation,
[43]
and in fact, NF1 mutations have been proposed as a source of resistance to BRAF inhibitors .
[20]
NF1-mutated tumors are clinically aggressive and have poor survival with very limited treatment options .
They tend to occur in sun-exposed skin in older male patients , and are also associated with desmoplastic
[19]
tumors, occurring more often in desmoplastic melanoma than any other NACM subtype [44,45] .
Triple-wild type
Triple-wild type NACM lacks any of the aforementioned mutations in BRAF, NRAS, or NF1 [Figure 2]. It is a
[20]
rare, heterogeneous group of cancers, with a predisposition for males between the ages of 60-70 . Interest-
ingly, it often harbors mutations in what are normally considered uveal melanoma drivers (discussed below),
such as GNA11, GNAQ, SF3B1, and KIT mutations [6,20,46] . Given the lack of a consistently present driver muta-
tion, triple-wild type NACM currently offers little promise for possible chemotherapy targets.