Page 11 - Read Online
P. 11
Lu et al. J Mater Inf 2022;2:11 I http://dx.doi.org/10.20517/jmi.2022.15 Page 7 of 18
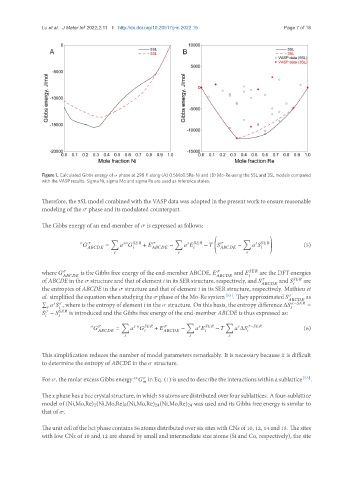
Figure 1. Calculated Gibbs energy of phase at 298 K along (A) 0.5Mo0.5Re-Ni and (B) Mo-Re using the 5SL and 3SL models compared
with the VASP results. Sigma Ni, sigma Mo and sigma Re are used as reference states.
Therefore, the 5SL model combined with the VASP data was adopted in the present work to ensure reasonable
modeling of the phase and its modulated counterpart.
The Gibbs energy of an end-member of is expressed as follows:
( )
∑ ∑ ∑
+ − (5)
= − −
where is the Gibbs free energy of the end-member ABCDE, and are the DFT energies
of ABCDE in the structure and that of element i in its SER structure, respectively, and and are
the entropies of ABCDE in the structure and that of element i in its SER structure, respectively. Mathieu et
al. simplified the equation when studying the phase of the Mo-Re system [53] . They approximated as
∑ , where is the entropy of element i in the structure. On this basis, the entropy difference Δ
−
=
− is introduced and the Gibbs free energy of the end-member ABCDE is thus expressed as:
∑ ∑ ∑
= + − Δ − (6)
−
This simplification reduces the number of model parameters remarkably. It is necessary because it is difficult
to determine the entropy of ABCDE in the structure.
For , the molar excess Gibbs energy in Eq. (1) is used to describe the interactions within a sublattice [53] .
The phasehasabcccrystalstructure,inwhich58atomsaredistributedoverfoursublattices. Afour-sublattice
model of (Ni,Mo,Re) 2(Ni,Mo,Re) 8(Ni,Mo,Re) 24(Ni,Mo,Re) 24 was used and its Gibbs free energy is similar to
that of .
Theunitcellofthebctphasecontains56atomsdistributedoversixsiteswithCNsof10, 12, 14and15. Thesites
with low CNs of 10 and 12 are shared by small and intermediate size atoms (Si and Co, respectively), the site