Page 57 - Read Online
P. 57
Liu et al. J Mater Inf 2022;2:20 https://dx.doi.org/10.20517/jmi.2022.29 Page 9 of 12
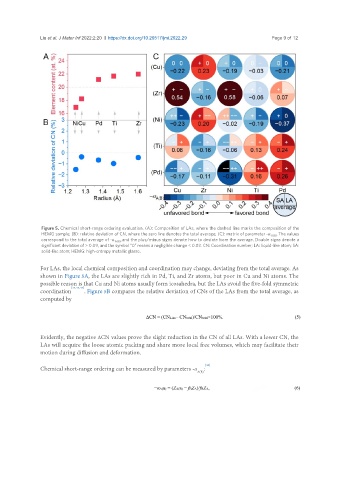
Figure 5. Chemical short-range ordering evaluation. (A): Composition of LAs, where the dashed line marks the composition of the
HEMG sample; (B): relative deviation of CN, where the zero line denotes the total average; (C): matrix of parameter -α A(B) . The values
correspond to the total average of -α A(B) , and the plus/minus signs denote how to deviate from the average. Double signs denote a
significant deviation of > 0.09, and the symbol “0” means a negligible change < 0.03. CN: Coordination number; LA: liquid-like atom; SA:
solid-like atom; HEMG: high-entropy metallic glasse.
For LAs, the local chemical composition and coordination may change, deviating from the total average. As
shown in Figure 5A, the LAs are slightly rich in Pd, Ti, and Zr atoms, but poor in Cu and Ni atoms. The
possible reason is that Cu and Ni atoms usually form icosahedra, but the LAs avoid the five-fold symmetric
coordination [34,43,44] . Figure 5B compares the relative deviation of CNs of the LAs from the total average, as
computed by
Evidently, the negative ΔCN values prove the slight reduction in the CN of all LAs. With a lower CN, the
LAs will acquire the loose atomic packing and share more local free volumes, which may facilitate their
motion during diffusion and deformation.
[45]
Chemical short-range ordering can be measured by parameters -α :
A(B)