Page 42 - Read Online
P. 42
Chen et al. J Mater Inf 2022;2:19 https://dx.doi.org/10.20517/jmi.2022.23 Page 15 of 21
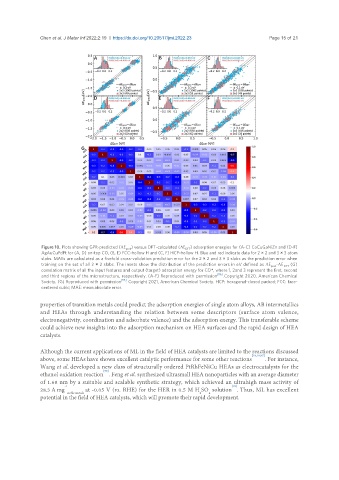
Figure 10. Plots showing GPR-predicted (ΔE pred ) versus DFT-calculated (ΔE DFT ) adsorption energies for (A-C) CoCuGaNiZn and (D-F)
AgAuCuPdPt for (A, D) on-top CO, (B, E) FCC-hollow H and (C, F) HCP-hollow H. Blue and red indicate data for 2 × 2 and 3 × 3 atom
slabs. MAEs are calculated as a fivefold cross-validation prediction error for the 2 × 2 and 3 × 3 slabs as the prediction error when
training on the set of all 2 × 2 slabs. The insets show the distribution of the prediction errors in eV defined as ΔE -ΔE . (G)
pred DFT
correlation matrix of all the input features and output (target) adsorption energy for CO*, where 1, 2and 3 represent the first, second
[86]
and third regions of the microstructure, respectively. (A-F) Reproduced with permission . Copyright 2020, American Chemical
[53]
Society. (G) Reproduced with permission . Copyright 2021, American Chemical Society. HCP: hexagonal-closed packed; FCC: face-
centered cubic; MAE: mean absolute error.
properties of transition metals could predict the adsorption energies of single atom alloys, AB intermetallics
and HEAs through understanding the relation between some descriptors (surface atom valence,
electronegativity, coordination and adsorbate valence) and the adsorption energy. This transferable scheme
could achieve new insights into the adsorption mechanism on HEA surfaces and the rapid design of HEA
catalysts.
Although the current applications of ML in the field of HEA catalysts are limited to the reactions discussed
above, some HEAs have shown excellent catalytic performance for some other reactions [18,19,97] . For instance,
Wang et al. developed a new class of structurally ordered PtRhFeNiCu HEAs as electrocatalysts for the
[98]
ethanol oxidation reaction . Feng et al. synthesized ultrasmall HEA nanoparticles with an average diameter
of 1.68 nm by a suitable and scalable synthetic strategy, which achieved an ultrahigh mass activity of
[99]
28.3 A mg -1 noble metals at -0.05 V (vs. RHE) for the HER in 0.5 M H SO solution . Thus, ML has excellent
2
4
potential in the field of HEA catalysts, which will promote their rapid development.