Page 410 - Read Online
P. 410
Gonzalez et al. Sunitinib effectiveness and safety in Costa Rica
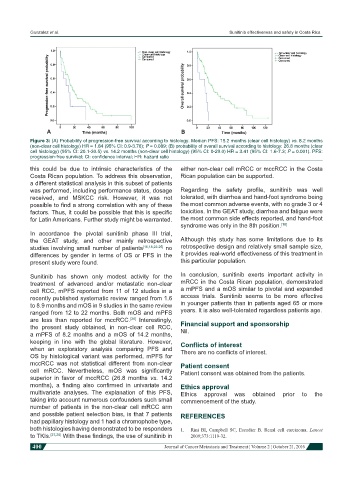
Figure 3: (A) Probability of progression-free survival according to histology. Median PFS: 15.2 months (clear cell histology) vs. 8.2 months
(non-clear cell histology) HR = 1.84 (95% CI: 0.9-3.76); P = 0.089; (B) probability of overall survival according to histology: 26.8 months (clear
cell histology) (95% CI: 20.1-30.5) vs. 14.2 months (non-clear cell histology) (95% CI: 0-29.0) HR = 3.41 (95% CI: 1.6-7.3; P = 0.001). PFS:
progression-free survival; CI: confidence interval; HR: hazard ratio
this could be due to intrinsic characteristics of the either non-clear cell mRCC or mccRCC in the Costa
Costa Rican population. To address this observation, Rican population can be supported.
a different statistical analysis in this subset of patients
was performed, including performance status, dosage Regarding the safety profile, sunitinib was well
received, and MSKCC risk. However, it was not tolerated, with diarrhea and hand-foot syndrome being
possible to find a strong correlation with any of these the most common adverse events, with no grade 3 or 4
factors. Thus, it could be possible that this is specific toxicities. In the GEAT study, diarrhea and fatigue were
for Latin Americans. Further study might be warranted. the most common side effects reported, and hand-foot
syndrome was only in the 8th position. [18]
In accordance the pivotal sunitinib phase III trial,
the GEAT study, and other mainly retrospective Although this study has some limitations due to its
studies involving small number of patients [16,18,22-25] no retrospective design and relatively small sample size,
differences by gender in terms of OS or PFS in the it provides real-world effectiveness of this treatment in
present study were found. this particular population.
Sunitinib has shown only modest activity for the In conclusion, sunitinib exerts important activity in
treatment of advanced and/or metastatic non-clear mRCC in the Costa Rican population, demonstrated
cell RCC, mPFS reported from 11 of 12 studies in a a mPFS and a mOS similar to pivotal and expanded
recently published systematic review ranged from 1.6 access trials. Sunitinib seems to be more effective
to 8.9 months and mOS in 9 studies in the same review in younger patients than in patients aged 65 or more
ranged from 12 to 22 months. Both mOS and mPFS years. It is also well-tolerated regardless patients age.
are less than reported for mccRCC. Interestingly, Financial support and sponsorship
[26]
the present study obtained, in non-clear cell RCC,
a mPFS of 8.2 months and a mOS of 14.2 months, Nil.
keeping in line with the global literature. However, Conflicts of interest
when an exploratory analysis comparing PFS and There are no conflicts of interest.
OS by histological variant was performed, mPFS for
mccRCC was not statistical different from non-clear Patient consent
cell mRCC. Nevertheless, mOS was significantly Patient consent was obtained from the patients.
superior in favor of mccRCC (26.8 months vs. 14.2
months), a finding also confirmed in univariate and Ethics approval
multivariate analyses. The explanation of this PFS, Ethics approval was obtained prior to the
taking into account numerous confounders such small commencement of the study.
number of patients in the non-clear cell mRCC arm
and possible patient selection bias, is that 7 patients REFERENCES
had papillary histology and 1 had a chromophobe type,
both histologies having demonstrated to be responders 1. Rini BI, Campbell SC, Escudier B. Renal cell carcinoma. Lancet
to TKIs. [27,28] With these findings, the use of sunitinib in 2009;373:1119-32.
400 Journal of Cancer Metastasis and Treatment ¦ Volume 2 ¦ October 21, 2016