Page 408 - Read Online
P. 408
Gonzalez et al. Sunitinib effectiveness and safety in Costa Rica
Table 1: Patient characteristics variant [Table 2]. However, a significant difference
All patients (n = 77) was found in mOS according to histological subtype
Median age (years, range) 58.9 (47.4-70.4) in favor of ccRCC when compared with non-clear cell
Patients older than 65 years (%) 25 (32.4) carcinoma: 26.8 months (95% CI: 20.1-30.5) vs. 14.2
Gender (%) months (95% CI: 0-29.0); HR: 3.41 (95% CI: 1.6-7.3;
Female 23 (29.9) P = 0.001) [Figure 3]. When univariate and multivariate
Male 54 (70.1)
ECOG/PS (%) analyses were performed, it was found that ccRCC was
0 60 (77.9) an independent prognostic factor in terms of OS but not
1 10 (12.9) PFS [Tables 3 and 4].
2 7 (9.2)
Histological variant (%) Sunitinib was, in general, well tolerated. There were
Clear cell carcinoma 67 (87.0) 17 patients (22%) who received a dose reduction to a
Papillary 7 (9.1) 37.5 mg daily schedule due to grade 1 or 2 toxicities;
Chromophobe 2 (2.6)
Collecting duct carcinoma 1 (1.3) no grade 3 or 4 toxicities were registered. Diarrhea and
MSKCC risk classification (%) hand-foot syndrome were the most commonly adverse
Low 47 (61.0) reactions described [Table 5].
Intermediate
25 (32.4)
High 5 (6.5) DISCUSSION
Site of metastasis (%)
Lung 55 (52.8) According to international RCC treatment guidelines,
Bone 19 (18.3) sunitinib is currently one of the preferred options
Liver 16 (15.3)
Central nervous system 10 (9.6) to treat metastatic clear cell renal cell carcinoma
Other 4 (3.8) (mccRCC). [14,15] Its efficacy and safety have been
ECOG: Eastern Cooperative Oncology Group; PS: performance evaluated in a phase III pivotal study and the global
status; MSKCC: Memorial Sloan Kettering Cancer Center expanded-access trial (GEAT). [16-18] There are few data
in Latin America regarding the effectiveness of sunitinib.
to those with less than 65 years. mPFS was 17.6 months In the GEAT trial, it was reported that a subset analysis
(95% CI: 10.2-25.0 months) vs. 8.2 months (95% CI: of 348 Latin American patients showed a mPFS and a
0.1-16.4 months); hazard ratio (HR): 1.93 (95% CI: 1.2- mOS of 12.1 and 16.9 months, respectively. [19,20] The
3.2); P = 0.011; mOS was 29.0 months (95% CI: 11.4- final analysis of this global trial including more than
46.5) vs. 19.0 months (95% CI: 11.0-26.9, HR = 1.82; 4,500 patients demonstrated a mPFS of 9.4 months
95% CI = 1.1-3.1); P = 0.022 [Figure 2]. These findings and a mOS of 18.7 months. In the present study we
[18]
were confirmed in univariate and multivariate analyses obtained a mPFS of 13.7 and a mOS of 21.0 months,
[Tables 3 and 4], showing that age was an independent very similar to the results reported globally. This strongly
prognostic factor either for PFS or OS. suggests that sunitinib has the same effectiveness in
the Latin American population as previously assessed
There was no difference in PFS by gender or histological in the pivotal trial and the GEAT, supporting the use of
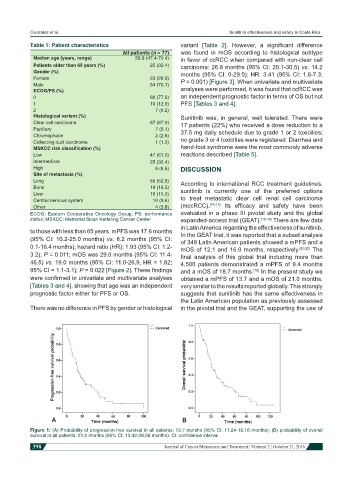
Figure 1: (A) Probability of progression-free survival in all patients: 13.7 months (95% CI: 11.24-16.16 months); (B) probability of overall
survival in all patients: 21.0 months (95% CI: 13.42-28.58 months). CI: confidence interval
398 Journal of Cancer Metastasis and Treatment ¦ Volume 2 ¦ October 21, 2016