Page 98 - Read Online
P. 98
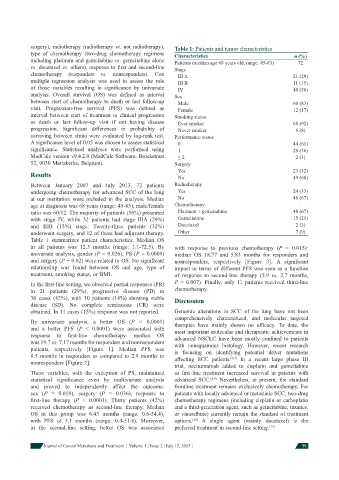
surgery), radiotherapy (radiotherapy vs. not radiotherapy), Table 1: Patients and tumor characteristics
type of chemotherapy (two-drug chemotherapy regimens Characteristics n (%)
including platinum and gemcitabine vs. gemcitabine alone Patients (median age 68 years old, range: 45-83) 72
vs. docetaxel vs. others), response to fi rst and second-line Stage
chemotherapy (responders vs. nonresponders). Cox III A 21 (29)
multiple regression analysis was used to assess the role III B 11 (15)
of those variables resulting in signifi cance by univariate IV 40 (56)
analysis. Overall survival (OS) was defi ned as interval Sex
between start of chemotherapy to death or last follow-up Male 60 (83)
visit. Progression-free survival (PFS) was defi ned as Female 12 (17)
interval between start of treatment to clinical progression Smoking status
or death or last follow-up visit if not having disease Ever smoker 66 (92)
progression. Signifi cant differences in probability of Never smoker 6 (8)
surviving between strata were evaluated by log-rank test. Performance status
A signifi cance level of 0.05 was chosen to assess statistical 0 44 (61)
signifi cance. Statistical analyses were performed using 1 26 (36)
MedCalc version v9.4.2.0 (MedCalc Software, Broekstraat ≥ 2 2 (3)
52, 9030 Mariakerke, Belgium). Surgery
Yes 23 (32)
Results No 49 (68)
Between January 2007 and July 2013, 72 patients Radiotherapy
undergoing chemotherapy for advanced SCC of the lung Yes 24 (33)
at our institution were included in the analysis. Median No 48 (67)
age at diagnosis was 68 years (range: 45-83); male/female Chemotherapy
ratio was 60/12. The majority of patients (56%) presented Platinum + gemcitabine 48 (67)
with stage IV, while 32 patients had stage IIIA (29%) Gemcitabine 15 (21)
and IIIB (15%) stage. Twenty-three patients (32%) Docetaxel 2 (3)
underwent surgery, and 12 of these had adjuvant therapy. Other 7 (9)
Table 1 summarizes patient characteristics. Median OS
in all patients was 12.3 months (range: 1.1-72.5). By with response to previous chemotherapy (P = 0.015):
univariate analysis, gender (P = 0.026), PS (P = 0.0009) median OS 18.77 and 5.83 months for responders and
and surgery (P = 0.02) were related to OS. No signifi cant nonresponders, respectively [Figure 3]. A signifi cant
relationship was found between OS and age, type of impact in terms of different PFS was seen as a function
treatment, smoking status, or BMI. of response to second-line therapy (5.9 vs. 2.7 months,
In the fi rst-line setting, we observed partial responses (PR) P = 0.007). Finally, only 11 patients received third-line
in 21 patients (29%), progressive disease (PD) in chemotherapy.
30 cases (42%), with 10 patients (14%) showing stable Discussion
disease (SD). No complete remissions (CR) were
obtained. In 11 cases (15%) response was not reported. Genomic alterations in SCC of the lung have not been
comprehensively characterized, and molecular targeted
By univariate analysis, a better OS (P < 0.0001) therapies have mainly shown no effi cacy. To date, the
and a better PFS (P < 0.0001) were associated with most important molecular and therapeutic achievements in
response to fi rst-line chemotherapy: median OS advanced NSCLC have been mostly confi ned to patients
was 19.7 vs. 7.17 months for responders and nonresponders with nonsquamous histology. However, recent research
patients, respectively [Figure 1]. Median PFS was is focusing on identifying potential driver mutations
8.5 months in responders as compared to 2.9 months in affecting SCC patients. [6,7] In a recent large phase III
nonresponders [Figure 2].
trial, necitumumab added to cisplatin and gemcitabine
These variables, with the exception of PS, maintained as fi rst-line treatment increased survival in patients with
statistical signifi cance even by multivariate analysis advanced SCC. [8,9] Nevertheless, at present, the standard
and proved to independently affect the outcome: frontline treatment remains exclusively chemotherapy. For
sex (P = 0.019), surgery (P = 0.036), response to patients with locally advanced or metastatic SCC, two-drug
fi rst-line therapy (P < 0.0001). Thirty patients (42%) chemotherapy regimens (including cisplatin or carboplatin
received chemotherapy as second-line therapy. Median and a third-generation agent, such as gemcitabine, taxanes,
OS in this group was 6.43 months (range: 0.6-54.4), or vinorelbine) currently remain the standard of treatment
with PFS of 3.1 months (range: 0.4-51.4). Moreover, options. A single agent (mainly docetaxel) is the
[10]
in the second-line setting, better OS was associated preferred treatment in second-line setting. [11]
Journal of Cancer Metastasis and Treatment ¦ Volume 1 ¦ Issue 2 ¦ July 15, 2015 ¦ 91