Page 44 - Read Online
P. 44
The early initial progression, occurring shortly after
the second adjuvant temozolomide cycle, made us
consider a scheme that could achieve a high rate of
disease control. The outstanding response obtained
at 4 months of treatment with fotemustine plus
bevacizumab, without radiological evidence from the
pre-existing tumor mass, prompted us to continue with
[12]
bevacizumab maintenance. When this patient had
received temozolomide monotherapy, he presented
with instability and vertiginous symptoms. During
a b
bevacizumab and fotemustine therapy, the neurological
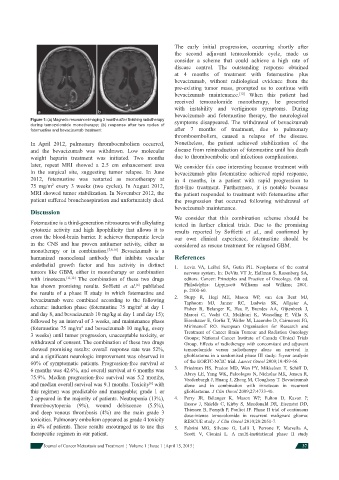
Figure 1: (a) Magnetic resonance imaging 3 months after fi nishing radiotherapy symptoms disappeared. The withdrawal of bevacizumab
during temozolomide monotherapy; (b) response after two cycles of
fotemustine and bevacizumab treatment after 7 months of treatment, due to pulmonary
thromboembolism, caused a relapse of the disease.
In April 2012, pulmonary thromboembolism occurred, Nonetheless, the patient achieved stabilization of the
and the bevacizumab was withdrawn. Low molecular disease from reintroduction of fotemustine until his death
weight heparin treatment was initiated. Two months due to thromboembolic and infectious complications.
later, repeat MRI showed a 2.5 cm enhancement area We consider this case interesting because treatment with
in the surgical site, suggesting tumor relapse. In June bevacizumab plus fotemustine achieved rapid response,
2012, fotemustine was restarted as monotherapy at in 4 months, in a patient with rapid progression to
2
75 mg/m every 3 weeks (two cycles). In August 2012, fi rst-line treatment. Furthermore, it is notable because
MRI showed tumor stabilization. In November 2012, the the patient responded to treatment with fotemustine after
patient suffered bronchoaspiration and unfortunately died. the progression that occurred following withdrawal of
bevacizumab maintenance.
Discussion
We consider that this combination scheme should be
Fotemustine is a third-generation nitrosourea with alkylating tested in further clinical trials. Due to the promising
cytotoxic activity and high lipophilicity that allows it to results reported by Soffi etti et al., and confi rmed by
cross the blood-brain barrier. It achieves therapeutic levels our own clinical experience, fotemustine should be
in the CNS and has proven antitumor activity, either as considered as rescue treatment for relapsed GBM.
monotherapy or in combination. [5,8-10] Bevacizumab is a
humanized monoclonal antibody that inhibits vascular References
endothelial growth factor and has activity in distinct 1. Levin VA, Leibel SA, Gutin PH . Neoplasms of the central
tumors like GBM, either in monotherapy or combination nervous system. In: DeVita VT Jr, Hellman S, Rosenberg SA,
with irinotecan. [11,12] The combination of these two drugs editors. Cancer: Principles and Practice of Oncology. 6th ed .
[6]
has shown promising results. Soffi etti et al. published Philadelphia: Lippincott Williams and Wilkins; 2001.
the results of a phase II study in which fotemustine and p. 2100-60.
bevacizumab were combined according to the following 2. Stupp R, Hegi ME, Mason WP, van den Bent MJ,
Taphoorn MJ, Janzer RC, Ludwin SK, Allgeier A,
2
scheme: induction phase ( fotemustine 75 mg/m at day 1 Fisher B, Belanger K, Hau P, Brandes AA, Gijtenbeek J,
and day 8, and bevacizumab 10 mg/kg at day 1 and day 15); Marosi C, Vecht CJ, Mokhtari K, Wesseling P, Villa S,
followed by an interval of 3 weeks, and maintenance phase Eisenhauer E, Gorlia T, Weller M, Lacombe D, Cairncross JG,
(fotemustine 75 mg/m and bevacizumab 10 mg/kg, every Mirimanoff RO. European Organisation for Research and
2
3 weeks) until tumor progression, unacceptable toxicity, or Treatment of Cancer Brain Tumour and Radiation Oncology
Groups; National Cancer Institute of Canada Clinical Trials
withdrawal of consent. The combination of these two drugs Group. Effects of radiotherapy with concomitant and adjuvant
showed promising results: overall response rate was 52%, temozolomide versus radiotherapy alone on survival in
and a signifi cant neurologic improvement was observed in glioblastoma in a randomised phase III study: 5-year analysis
60% of symptomatic patients. Progression-free survival at of the EORTC-NCIC trial. Lancet Oncol 2009;10:459-66.
6 months was 42.6%, and overall survival at 6 months was 3. Friedman HS, Prados MD, Wen PY, Mikkelsen T, Schiff D,
75.9%. Median progression-free survival was 5.2 months, Abrey LE, Yung WK, Paleologos N, Nicholas MK, Jensen R,
Vredenburgh J, Huang J, Zheng M, Cloughesy T. Bevacizumab
and median overall survival was 9.1 months. Toxicity with alone and in combination with irinotecan in recurrent
[6]
this regimen was predictable and manageable; grade 1 or glioblastoma. J Clin Oncol 2009;27:4733-40.
2 appeared in the majority of patients. Neutropenia (13%), 4. Perry JR, Bélanger K, Mason WP, Fulton D, Kavan P,
thrombocytopenia (9%), wound dehiscence (5.5%), Easaw J, Shields C, Kirby S, Macdonald DR, Eisenstat DD,
and deep venous thrombosis (4%) are the main grade 3 Thiessen B, Forsyth P, Pouliot JF. Phase II trial of continuous
dose-intense temozolomide in recurrent malignant glioma:
toxicities. Pulmonary embolism appeared as grade 4 toxicity RESCUE study. J Clin Oncol 2010;28:2051-7.
in 4% of patients. These results encouraged us to use this 5. Fabrini MG, Silvano G, Lolli I, Perrone F, Marsella A,
therapeutic regimen in our patient. Scotti V, Cionini L. A multi-institutional phase II study
Journal of Cancer Metastasis and Treatment ¦ Volume 1 ¦ Issue 1 ¦ April 15, 2015 ¦ 37