Page 95 - Read Online
P. 95
Ma et al. J Cancer Metastasis Treat 2022;8:25 https://dx.doi.org/10.20517/2394-4722.2022.17 Page 13 of 20
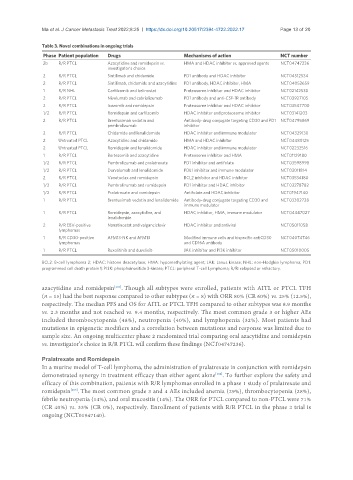
Table 3. Novel combinations in ongoing trials
Phase Patient population Drugs Mechanisms of action NCT number
2b R/R PTCL Azacytidine and romidepsin vs. HMA and HDAC inhibitor vs. approved agents NCT04747236
investigator’s choice
2 R/R PTCL Sintilimab and chidamide PD1 antibody and HDAC inhibitor NCT04512534
2 R/R PTCL Sintilimab, chidamide and azacytidine PD1 antibody, HDAC inhibitor, HMA NCT04052659
1 R/R NHL Carfilzomib and belinostat Proteosome inhibitor and HDAC inhibitor NCT02142530
2 R/R PTCL Nivolumab and cabrializumab PD1 antibody and anti-CSF-1R antibody NCT03927105
2 R/R PTCL Ixazomib and romidepsin Proteosome inhibitor and HDAC inhibitor NCT03547700
1/2 R/R PTCL Romidepsin and carfilzomib HDAC inhibitor and proteosome inhibitor NCT03141203
2 R/R PTCL Brentuximab vedotin and Antibody-drug conjugate targeting CD30 and PD1 NCT04795869
pembrolizumab inhibitor
2 R/R PTCL Chidamide and lenalidomide HDAC inhibitor and immune modulator NCT04329130
2 Untreated PTCL Azacytidine and chidamide HMA and HDAC inhibitor NCT04480125
2 Untreated PTCL Romidepsin and lenalidomide HDAC inhibitor and immune modulator NCT02232516
1 R/R PTCL Bortezomib and azacytidine Proteosome inhibitor and HMA NCT01129180
1/2 R/R PTCL Pembrolizumab and pralatrexate PD1 inhibitor and antifolate NCT03598998
1/2 R/R PTCL Durvalumab and lenalidomide PDL1 inhibitor and immune modulator NCT03011814
2 R/R PTCL Venetoclax and romidepsin BCL2 inhibitor and HDAC inhibitor NCT03534180
1/2 R/R PTCL Pembrolizumab and romidepsin PD1 inhibitor and HDAC inhibitor NCT03278782
1/2 R/R PTCL Pralatrexate and romidepsin Antifolate and HDAC inhibitor NCT01947140
1 R/R PTCL Brentuximab vedotin and lenalidomide Antibody-drug conjugate targeting CD30 and NCT03302728
immune modulator
1 R/R PTCL Romidepsin, azacytidine, and HDAC inhibitor, HMA, immune modulator NCT04447027
lenalidomide
2 R/R EBV-positive Nanatinostat and valganciclovir HDAC inhibitor and antiviral NCT05011058
lymphomas
1 R/R CD30-positive AFM13-NK and AFM13 Modified immune cells and bispecific antiCD30 NCT04074746
lymphomas and CD16A antibody
1 R/R PTCL Ruxolitinib and duvelisib JAK inhibitor and PI3K inhibitor NCT05010005
BCL2: B-cell lymphoma 2; HDAC: histone deacetylase; HMA: hypomethylating agent; JAK: Janus kinase; NHL: non-Hodgkin lymphoma; PD1:
programmed cell death protein 1; PI3K: phosphoinositide 3-kinase; PTCL: peripheral T-cell Lymphoma; R/R: relapsed or refractory.
azacytidine and romidepsin . Though all subtypes were enrolled, patients with AITL or PTCL TFH
[103]
(n = 15) had the best response compared to other subtypes (n = 8) with ORR 80% (CR 60%) vs. 25% (12.5%),
respectively. The median PFS and OS for AITL or PTCL TFH compared to other subtypes was 8.9 months
vs. 2.3 months and not reached vs. 9.4 months, respectively. The most common grade 3 or higher AEs
included thrombocytopenia (48%), neutropenia (40%), and lymphopenia (32%). Most patients had
mutations in epigenetic modifiers and a correlation between mutations and response was limited due to
sample size. An ongoing multicenter phase 2 randomized trial comparing oral azacytidine and romidepsin
vs. investigator’s choice in R/R PTCL will confirm these findings (NCT04747236).
Pralatrexate and Romidepsin
In a murine model of T-cell lymphoma, the administration of pralatrexate in conjunction with romidepsin
demonstrated synergy in treatment efficacy than either agent alone . To further explore the safety and
[104]
efficacy of this combination, patients with R/R lymphomas enrolled in a phase 1 study of pralatrexate and
[105]
romidepsin . The most common grade 3 and 4 AEs included anemia (29%), thrombocytopenia (28%),
febrile neutropenia (14%), and oral mucositis (14%). The ORR for PTCL compared to non-PTCL were 71%
(CR 40%) vs. 33% (CR 0%), respectively. Enrollment of patients with R/R PTCL in the phase 2 trial is
ongoing (NCT01947140).